Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
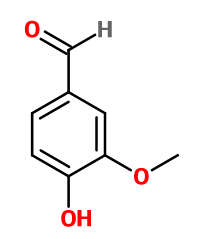
Vanilline - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
Vanillin Natural (ex-Clove Oil) | VN-0301 |
Visit website
|
Natural |



|
100 | Eugenia caryophyllus | Clove Oil | Indonesia | 100 Kgs |
General Presentation
-
CAS N° : 121-33-5
-
EINECS number : 204-465-2
-
FEMA number : 3107
-
FLAVIS number : 05.018
-
JECFA number : 889
-
Appearance : White solid
-
Density : 1,23
-
Volatility : Heart/Base
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C8H8O3
-
Molecular Weight : 152,15 g/mol
-
Log P : 1,23
-
Fusion Point : 83°C
-
Boiling Point : 285°C
-
Detection Threshold : 29 ppb à 1,6 ppm (0,00016%) selon les personnes, Son seuil de reconnaissance est quant à lui d'environ 4 ppm
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 153°C
Uses
Uses in perfumery :
Vanillin is used in reconstitutions of vanilla, in oriental perfumes and in gourmet notes. Used to bring a sweet and milky effect to a fruity, floral, spicy or woody composition.
Year of discovery :
Discovered in 1874, by chemists Tiemann and Haarmann, by hydrolysis of coniferin with the enzyme emulsin, followed by oxydation of the obtained alcohol with potassium dichromate and sulfuric acid.
Natural availability :
Natural Vanillin is not very much produced, as it has to be extracted from Vanilla Bourbon Absolute (or other origins), which has already a very expensive process of manufacturing. Nevertheless, new processes to obtain biochemical Vanillin allows to obtain a vanillin that can be considered as natural.
Isomerism :
Vanillin is a constitutional isomer of Methyl Salicylate. Although close structurally, Methyl Salicylate has a much fruitier, more floral and powerful smell than Vanillin.
Synthesis precursor :
Vanillin is a precursor to the synthesis of several compounds of olfactory interest. Addition reactions can be carried out on its aromatic ring. A catalytic hydrogenation allows to synthesize Vanillic Alcohol. Several esterifications are possible by exploiting the alcohol function of the molecule (as in the synthesis of Isobutavan®), as well as aldol condensation by exploiting the aldehyde function.
Synthesis route :
Vanillin is mainly synthesized from lignin or Guaiacol. Since these processes result in 160 kg of waste per kg of finished product, the synthesis of vanillin tends to be replaced by biosynthetic pathways, from rice bran, natural curcumin or natural Eugenol. The synthesis from lignin is made by an alklaine treatment at high temperature and pressure with oxidants. Vanillin is separated from the other compounds obtained by extraction, distillation and crystallization. Another synthetic route starts from Guaiacol, synthesized from Catechol. Guaiacol condenses to glyoxylic acid in a alklaine medium and at room temperature. Vanillin is obtained by acidification and decarboxylation of the reaction mixture. Another similar process uses Guaiacol and formaldehyde, to obtain the ortho and para-vanillic alcohols. Then, a catalytic oxidation (with palladium and bismuth) allows to obtain Vanillin, separated from the other reaction products by crystallization. Using an excess of formaldehyde involves an oxidation and decarboxylation step, always to obtain Vanillin as the final product. Nowadays, several production processes using biochemistry consist in a bioconversion of ferulic acid, Isoeugenol or Eugenol, using bacteria, fungi and plant cells capable of transforming these compounds.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

