Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
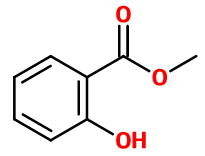
Salicylate de Méthyle - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 119-36-8
-
EINECS number : 204-317-7
-
FEMA number : 2745
-
FLAVIS number : 09.749
-
JECFA number : 899
-
Appearance : Colorless liquid
-
Density : 1,174
-
Volatility : Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C8H8O3
-
Molecular Weight : 152,15 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : -7°C
-
Boiling Point : 222°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 75°C
Uses
Uses in perfumery :
Used in tuberose and orange blossom notes, as a solar note modifier, bringing a fruity nuance.
Year of discovery :
Data not available.
Natural availability :
Methyl Salicylate is the main component of Wintergreen EO. This raw material obtained from wintergreen leaves is used to extract methyl salicylate. Most often, it is natural methyl salicylate that is used in perfumery. It is also found in Tuberose Absolute, Ylang-Ylang Extra EO (and other ylang fractions) and Birch Rectified EO.
Isomerism :
Vanillin is a constitutional isomer of Methyl Salicylate. The two structures of these molecules are also quite linked, although Vanillin has an ether group and an aldehyde group instead of an ester group. Its smell is sweeter, less powerful and reminiscent of that of Vanilla Bourbon Absolute.
Synthesis precursor :
Methyl Salicylate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Methyl Salicylate is often obtained on a natural way. However, it can be synthesized by an esterification reaction. This reaction involves salicylic acid with methanol, in the presence of a small amount of a concentrated strong acid such as sulphuric acid. The yield of this reaction can be improved by using salicylic anhydride or chloro-salicylic acid instead of salicylic acid.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
Methyl salicylate must not be used in finished products intended for children under the age of 6, except for toothpaste.
Additionally, it is subject to different labelling thresholds depending on product type:
• Leave-on skin products (excluding facial makeup, body lotions in spray/aerosol form, aerosol deodorants and hydroalcoholic perfumes), as well as leave-on hair and facial hair products (excluding sprays/aerosols): 0.06%
• Facial makeup (excluding lip products, eye makeup and makeup removers): 0.05%
• Eye makeup and makeup removers: 0.002%
• Leave-on hair and facial hair sprays/aerosols: 0.009%
• Aerosol deodorants: 0.003%
• Body lotions in spray/aerosol form: 0.04%
• Rinse-off skin products (excluding hand cleansers), and rinse-off hair and facial hair products: 0.06%
• Hand washing products: 0.6%
• Hydroalcoholic perfumes: 0.6%
• Lip products: 0.03%
IFRA 51th :
This ingredient is not restricted for the 51th amendment

