Photo credits: ScenTree SAS
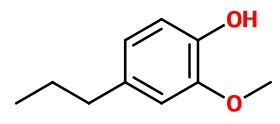
Dihydroeugenol
2-methoxy-4-propylphenol ; 4-propylguaiacol ; Ortho-5-propylhydroxyanisole ; Ortho-4-propylmethoxyphenol ; Cerulignol ; Coerulignol ; Guaiacylpropane ; Cerulignol ; Cerulignol ; Dihydro eugenol ; Guaiacyl propane ; 4-hydroxy-3-methoxypropyl benzene ; (4-hydroxy-3-methoxyphenyl) propane ; 4-propyl guaiacol ; Para-propyl guaiacol ; 5-propyl ortho-hydroxyanisole ; 4- propyl-2-methoxyphenol

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Dihydroeugenol | CL-804 |
Visit website
|
Natural |



|
100 | Eugenia caryophyllus | Clove Oil | Indonesia | 100 Kgs |
General Presentation
-
CAS N° : 2785-87-7
-
EINECS number : 220-499-0
-
FEMA number : 3598
-
FLAVIS number : 04.049
-
JECFA number : 717
-
Appearance : Pale yellow liquid
-
Density : 1,038
-
Volatility : Head/Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C10H14O2
-
Molecular Weight : 166,22 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : 17°C
-
Boiling Point : 240°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 113°C
Uses
Uses in perfumery :
DihydroEugenol is used to replace Eugenol is some accords, as it is not a regulated ingredient, for the same reasons as Eugenol, but mostly when the aim is to bring more facets to fresh spices such as black pepper, cardamom etc.
Year of discovery :
Data not available.
Natural availability :
DihydroEugenol has been identified in diverse consumer goods such as some whiskeys and cheeses. Nevertheless, it is not extracted from any plant, to get it on a natual way.
Isomerism :
DihydroEugenol is a constitutional isomer of Viridine®, even if it doesn't have the same smell at all.
Synthesis precursor :
DihydroEugenol is not a precursor to the synthesis of any other molecule used in perfumery.
Synthesis route :
DihydroEugenol can be synthetised starting with Isoeugenol or Eugenol, using a catalytical hydrogenation reaction. This reaction consists in putting the molecule under a large pressure of hydrogen, in the presence of a metallic catalyst on carbon, such as platinium or palladium. The insaturation present in Isoeugenol or Eugenol is transformed into a simple bond in the final product.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,13 % 0,039 % 0,78 % 0,73 % 0,19 % 0,19 % 0,19 % 0,062 %0,43 % Cat.5A B C DCat.6 0,19 % 0,19 % 0,19 % 0,062 %0,43 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 1,5 % 1,5 %0,062 % 1,4 % 1,4 % 5,1 %0,062 % 0,062 %No Restriction Cat.10A BCat.11A BCat.12 1,4 % 5,1 %0,062 % 0,062 %No Restriction


