Photo credits: ScenTree SAS
Viridine®
PADMA (PhenylAcetaldehyde Dimethylacetal) ; 2,2-dimethoxyethylbenzene ; 2,2-dimethoxy-1-phenyl ethane ; 1,1-dimethoxy-2-phenylethane ; Hyscylene p ; Phenacetaldehyde dimethyl acetal ; Rosal ; Vert de lilas ; Alpha-tolyl aldehyde dimethyl acetal

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Phenylacetaldehyde DMA | 2311172010 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | China | - |
General Presentation
-
CAS N° : 101-48-4
-
EINECS number : 202-945-6
-
FEMA number : 2876
-
FLAVIS number : 06.006
-
JECFA number : 1003
-
Appearance : Colorless liquid
-
Density : 1,004
-
Volatility : Head/Heart
-
Price Range : €
Physico-chemical properties
-
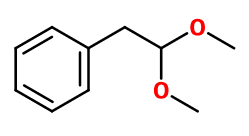
Molecular formula : C10H14O2
-
Molecular Weight : 166,22 g/mol
-
Log P : 1,9
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 220°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 88°C
Uses
Uses in perfumery :
Viridine® is used in rose, hyacinth, honeysuckle, lily of the valley, lilac, carnation, gardenia, lily and geranium notes. Useful in woody-patchouli and vetiver notes. Brings a ripe fruit effect to fruit accords. Also used in eaux fraiches and in fougere perfumes.
Year of discovery :
Data not available.
Natural availability :
Viridine® is not available in its natural state.
Isomerism :
Viridine® is a constitutional isomer of DihydroEugenol, even if both molecules do not have the same smell.
Synthesis precursor :
Viridine® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Viridine® is synthesized by an acetalization reaction between Phenylacetic Aldehyde (synthesized from styrene oxide for example) and methanol. This reaction is catalysed by a strong concentrated acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

