Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Velvione® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 37609-25-9
-
EINECS number : 253-568-9
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid that crystallizes at room temperature
-
Density : 0,926
-
Volatility : Base
-
Price Range : €€€€
Physico-chemical properties
-
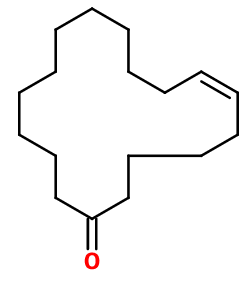
Molecular formula : C16H28O
-
Molecular Weight : 236,4 g/mol
-
Log P : >6,00
-
Fusion Point : 21°C
-
Boiling Point : 300°C
-
Detection Threshold : 0,6 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 160°C
Uses
Uses in perfumery :
Velvione® is used in floral notes, especially white and solar, and in feminine notes in general, to provide tenacity and trail.
Year of discovery :
1970
Natural availability :
Velvione® is not available in its natural state.
Isomerism :
Velvione® used in perfumery is a 60/40 mixture of its trans/cis isomers. There is no enantiomerically pure version marketed. In addition, Velvione® is a constitutional isomer of Muscenone®. Their structure remains fairly close, and can explain the musky aspect they share.
Synthesis precursor :
Velvione® does not synthesize other compounds of olfactory interest.
Synthesis route :
Velvione® is synthesized in three steps, starting from cyclododecanone. This molecule is able to react with chlorine under pressure to obtain 2-chlorocyclododecanone, formed in priority. Then, this compound reacts with two molar equivalents of vinyl magnesium chloride to give 1,2-divinyl-cyclododecan-1-ol. By an Oxy-Cope rearrangement, Velvione® forms by itself (this rearrangement reorganizes the skeleton of certain unsaturated alcohols). The conversion of alcohol to an alcoholate accelerates the rearrangement. In this last step, the alcohol group becomes the ketone group of Velvione®.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

