Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Muscenone - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 82356-51-2
-
EINECS number : 429-900-5
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,93
-
Volatility : Base
-
Price Range : €€€€
Physico-chemical properties
-
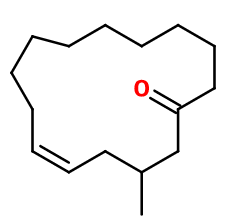
Molecular formula : C16H28O
-
Molecular Weight : 236,39 g/mol
-
Log P : 4,88
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >100°C (>212°F)
Uses
Uses in perfumery :
Muscenone® is used only in fine fragrance for all types of accords, especially musky, leather, chypre, fairly heavy and warm notes.
Gives volume when mixed with other musks.
Year of discovery :
Discovered in 1967. 'Muscenone®' trademark has been published and protected by Firmenich SA since 28/01/2011 (brand N°1069869)
Natural availability :
Muscenone® is not available in its natural state.
Isomerism :
The molecule has a double bond that gives rise to two possible diastereoisomers with similar smells. It is the mixture of the two isomers that is used in perfumery. Sandela®, Cedramber® and Ambroxan® are constitutional isomers of Muscenone®. Their smell is however very different, as it is woody and ambery, instead of musky. Velvione®, on the other hand, has a structure that is relatively similar to Muscenone® and is its constitutional isomer. This explains the musky aspect shared by both molecules.
Synthesis precursor :
Muscenone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Muscenone® is a macrocyclic musk synthesized from cyclododecanone. A synthesis in several steps allows to synthesize 14-methyl-16-oxabicyclo [10.3.1] hexadec-1(15)-ene. This compound can finally be isomerized in the presence of phosphoric acid to give the racemic mixture of Muscenone®.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

