Photo credits: ScenTree SAS
Terpineol
α,4-trimethyl-(1S)-3-cyclohexene-1-methanol ; α,α,4-trimethyl-(1R)-3-cyclohexene-1-methanol ; 1-methyl-4-(1-methylethylidene)-cyclohexanol ; p-Menth-1-en-8-ol ; 2-(4-Methylcyclohex-3-en-1-yl)propan-2-ol

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 8000-41-7
-
EINECS number : 202-680-6
-
FEMA number : 3045
-
FLAVIS number : 02.014
-
JECFA number : 366
-
Appearance : Colorless liquid
-
Density : 0,93
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C10H18O
-
Molecular Weight : 154,25 g/mol
-
Log P : 2,91
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 214–217°C (417–423 °F)
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 89°C (192°F)
Uses
Uses in perfumery :
Besides the fact that terpineol is widely used for the synthesis of other olfactive ingredients, it can also be used as such in our formulas. Its refreshing pine-like odor is widely used in the detergent and home care industry as well as for creating olfactory reconstitutions and bases. It is often detected in chromatography, because this ingredient is found in a large number of essential oils.
Year of discovery :
Terpineol's structure determined in 1885 by Wallach, Tiemann and Semler.
Natural availability :
Terpineol can be obtained from the essential oil of many plants by fractional distillation, such as Turpentine EO, Lavender EO, Wormwood EO, Cardamom EO or Clary Sage EO. It is therefore posible to have a natural quality. This ingredient is found, in large quantities or in trace, in a very large quantity of ingredients used in our fragrances. Some references identify more than 200 oils containing it.
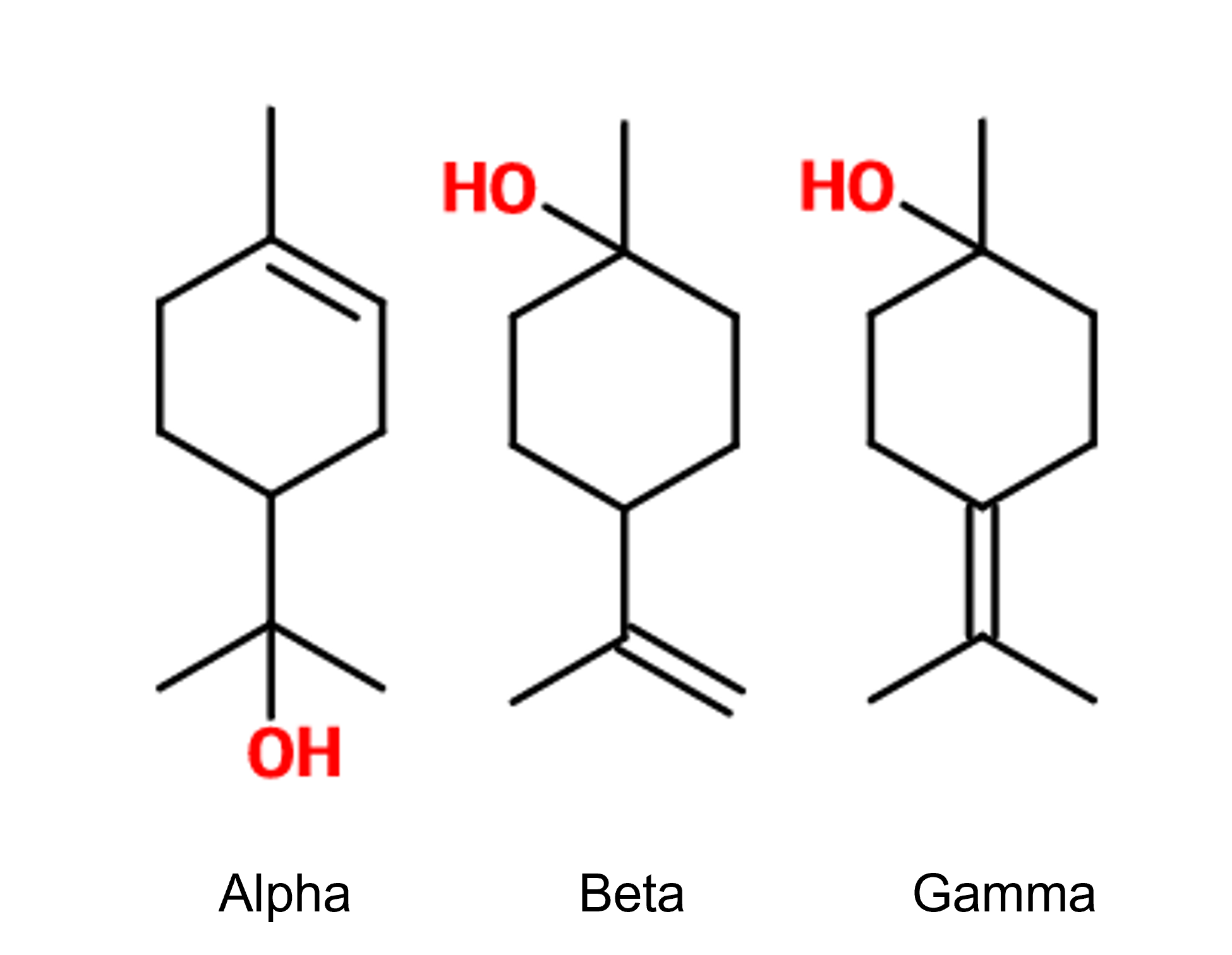
Isomerism :
Terpineol is a mixture of isomers. There are four of them. The alpha-terpineol ; beta-terpineol ; gamma-terpineol as well as terpinen-4-ol.
Synthesis precursor :
Terpineol is a precursor for the synthesis of a large number of olfactory compounds.
Synthesis route :
There are several ways to obtain Terpineol. Either by isolation from a natural ingredient, or by synthesis. The most commonly used reaction mechanisms start with Turpentine EO, alpha-pinene or D-Limonene
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

