Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
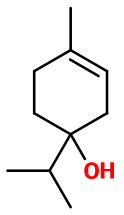
General Presentation
-
CAS N° : 562-74-3
-
EINECS number : 209-235-5
-
FEMA number : 2248
-
FLAVIS number : 02.072
-
JECFA number : 439
-
Appearance : Colorless liquid
-
Density : 0,931
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C10H18O
-
Molecular Weight : 154,25 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 89°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 79°C
Uses
Uses in perfumery :
Terpinen-4-ol is used for aromatic accords, around pine, pepper and geranium, especially in detergents and functional perfumes. It brings an earthy effect.
Year of discovery :
Data not available.
Natural availability :
Terpinen-4-ol is present in many essential oils, especially aromatic ones. It is found in a large quantity in Marjoram EO and in Tea Tree EO. It is present in moderate quantities in Lavender EO, Nutmeg EO and Blackcurrant Bud Absolute.
Isomerism :
Terpinen-4-ol used in perfumery, and often found in nature, is a racemic mixture of its two enantiomers, dextrorotatory and levorotatory. Both have an equivalent odor, which explains the use of the mixture of the two isomers. Terpinen-4-ol is also a positional isomer of Alpha-Terpineol, which has a more floral and less earthy odor.
Synthesis precursor :
Terpinen-4-ol can be used for the synthesis of Alpha-Terpineol. The goal of this synthesis is to shift the alcohol function of the initial molecule to its neighbouring carbon. However, this synthesis is not very common.
Synthesis route :
Terpinen-4-ol is often synthesized at a low cost as a by-product in the synthesis of Alpha-Terpineol from Terpine hydrate. The resulting molecule is not completely pure. Nevertheless, it is possible to synthesize pure Terpinen-4-ol from Terpinolene by photosensitized oxidation, followed by reduction of the intermediary peroxide, and selective hydrogenation of the intermediate alcohol formed.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

