Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Sandela® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 3407-42-9
-
EINECS number : 222-294-1
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless viscous liquid
-
Density : 0,972
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C16H28O
-
Molecular Weight : 236,4 g/mol
-
Log P : 5,27
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 160°C (à4 hPa)
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 152°C
Uses
Uses in perfumery :
Sandela® is used in woody notes and sandalwood reproductions. To be combined with Sandalore for more facets. This material is often 85% solubilized in Isopropyl Myristate before dilution in alcohol.
Year of discovery :
1942
Natural availability :
Sandela® is not available in its natural state.
Isomerism :
Sandela® is the only one of the isomers resulting from the synthesis described above to be odorous and to present a real interest for perfumery. Ambroxan®, Cedramber® and Muscenone® are constitutional isomers of Sandela®. Their smell can also be woody, or even musky. It is very different anyway.
Synthesis precursor :
Sandela® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
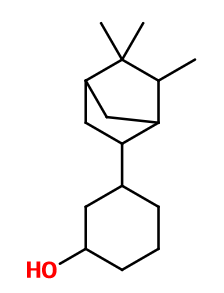
Sandela® is part of a mixture of isomers synthesized by reaction between Camphene and Guaiacol, in the presence of a Lewis acid such as boron trifluoride. During this first stage, the Camphene undergoes a rearrangement in three forms: Isocamphyl, Isofenchyl and Isobornyl, which gives rise to the mixture of isomers. A high temperature catalytic hydrogenation of these derivatives allows to obtain a new mixture with more isomers. Indeed, the terpenic half of the final molecule may be in axial or equatorial position with respect to the other half of the molecule. 3-trans-Isocamphylcyclohexanol, corresponding to Sandela®, is one of the isomers of the mixture. It is isolated by a fractional distillation. Another method of synthesis starts from Cathecol rather than Guaiacol, and allows to obtain a greater proportion of Sandela® in the final mixture (especially if the hydrogenation step is made under high pressure and catalysed with cobalt).
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

