Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Prenyl Acetate BMBcert™ | 30786722 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Prenylacetate | 30456214 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 1191-16-8
-
EINECS number : 214-730-4
-
FEMA number : 4202
-
FLAVIS number : 09.692
-
JECFA number : 1827
-
Appearance : Colorless liquid
-
Density : 0,917
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
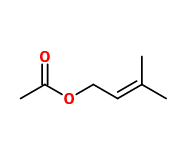
Molecular formula : C7H12O2
-
Molecular Weight : 128,17 g/mol
-
Log P : 1,7
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 152°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 49°C
Uses
Uses in perfumery :
Prenyl acetate is used for banana and red fruit accords, associated with floral notes to add a fruity aspect and to boost the top note.
Year of discovery :
Data not available.
Natural availability :
Prenyl acetate is slightly found (less than 1%) in Ylang-Ylang distillation fractions (Ylang-Ylang Extra EO for example). It can be extracted from it on a natural state.
Isomerism :
Prenyl acetate is a constitutional isomer of cis-3-Hexenyl Formate. The smell of Prenyl acetate also evokes pear fruit and has a green nuance, much less important than in cis-3-Hexenyl Formate.
Synthesis precursor :
Prenyl acetate is not a precursor to the synthesis of another compound of olfactive interest.
Synthesis route :
Prenyl acetate is synthesized by an esterification reaction between acetic acid and prenol (or 3-methyl-2-butenol). This synthesis can be optimized to get a better yield, replacing acetic acid by acetic anhydride or chloroacetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

