Photo credits: ScenTree SAS
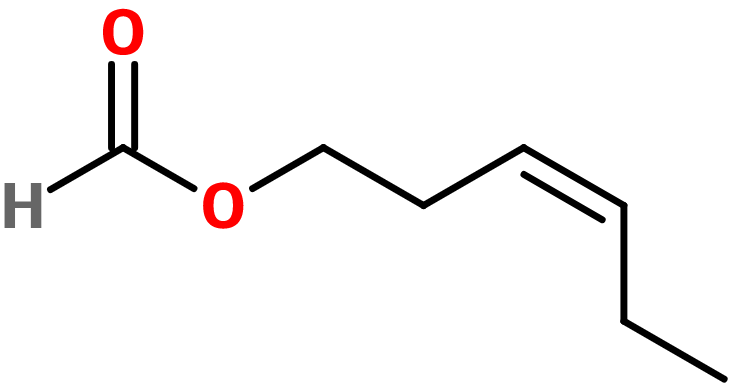
Cis-3-hexenyl Formate
Cis-3-Hexenyl formiate ; Cis-3-hexen-1-yl formiate ; Cis-3-hexen-1-yl formate ; Cis-3-hexen-1-yl formiate ; Cis-hex-3-enyl formate ; Cis-hex-3-enyl formiate ; Cis-hex-3-en-1-yl formate ; Cis-hex-3-en-1-yl formiate ; (Z)-3-hexen-1-yl formate ; (Z)-3-hexen-1-yl formiate

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 33467-73-1
-
EINECS number : 251-532-7
-
FEMA number : 3353
-
FLAVIS number : 09.240
-
JECFA number : 123
-
Appearance : Colorless liquid
-
Density : 0,91
-
Volatility : Head/Heart
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C7H12O2
-
Molecular Weight : 128,17 g/mol
-
Log P : 2,07
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 157°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 45°C
Uses
Uses in perfumery :
Cis-3-Hexenyl Formate is used in small quantities in some perfumes to give a juicy aspect to an apple or pear note. It can be used in combination with other cis-3-Hexenol derivatives to give many effects and bring roundness to fruity notes. It can be used with Galbanum EO and Violet Leaf Absolute notes.
Year of discovery :
Data not available.
Natural availability :
Cis-3-Hexenyl Formate can be extracted from Corn Mint EO, and is found in raspberry and mango in trace amounts.
Isomerism :
Trans-3-Hexenyl Formate, the cis-3-Hexenyl Formate diastereoisomer, is almost not used in perfumes. One positional isomer called trans-2-Hexenyl Formate is sometimes used for its rummy green apple note.
Synthesis precursor :
Cis-3-Hexenyl Formate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Cis-3-Hexenyl Formiate results from an esterification of cis-3-Hexenol with formic acid (or methanoic acid), in the presence of an acid catalysor as concentrated sulfuric acid. Using formic anhydride or chloromethanoic acid can also enhance the yield of this reaction.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

