Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 22629-49-8
-
EINECS number : 245-142-6
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Pale yellow liquid
-
Density : 0,84
-
Volatility : Base
-
Price Range : €€€
Physico-chemical properties
-
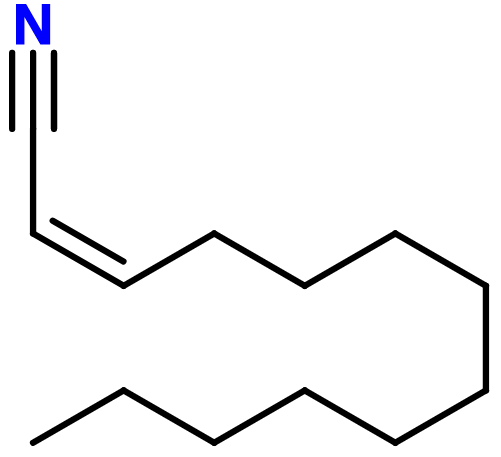
Molecular formula : C13H23N
-
Molecular Weight : 193,33 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 289°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >93°C
Uses
Uses in perfumery :
Ozonil® is used in zesty notes, to bring an aldehidic top note and a marine, ozonic and voluminous base. It is also used to bring a zesty nuance to marine notes.
Year of discovery :
Data not available.
Natural availability :
Ozonil® is not reported as found in nature, and can thus not be extracted from any plant.
Isomerism :
Ozonil® has one double bond giving birth to two possible diastereoisomers. In perfumery, a mixture of these two isomers is used.
Synthesis precursor :
Ozonil® is not a precursor for the synthesis of another material used in perfumery.
Synthesis route :
Ozonil® is prepared by a Knoevenagel condensation between Aldehyde C-11 Undecylic and cyanoacetic acid. A decarboxylation reaction (use of high heat and decarboxylase enzym) is then carried out to form the alcene function of the final product, Ozonil®.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

