Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
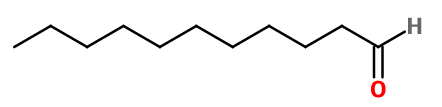
Aldéhyde C11 Undécylique - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 112-44-7
-
EINECS number : 203-972-6
-
FEMA number : 3092
-
FLAVIS number : 05.034
-
JECFA number : 107
-
Appearance : Colorless liquid
-
Density : 0,828
-
Volatility : Head
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C11H22O
-
Molecular Weight : 168,28 g/mol
-
Log P : 3,84
-
Fusion Point : -10°C
-
Boiling Point : 225°C
-
Detection Threshold : 2,9616 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 105°C
Uses
Uses in perfumery :
Aldehyde C-11 Undecylic is used in all types of compositions to provide a typical aldehydic facet. Particularly used in citrus accords and floral-aldehydic notes to bring this characteristic note.
Year of discovery :
Data not available.
Natural availability :
Aldehyde C-11 is present in Cordiander Leaf EO, in trace amounts in the essential oil of certain citrus fruits such as in Bergamot EO or Mandarin Yellow EO and Narcissus Absolute. It can be extracted from Coriander Leaf EO.
Isomerism :
Undecavertol® is a constitutional isomer of Aldehyde C-11 undecylic. Its smell is however very different.
Synthesis precursor :
Aldehyde C-11 undecylic forms a Schiff base by reaction with Methyl Anthranilate or Indole, for example. It can also be used for condensation with another aldehyde or ketone to form the desired alcene. It is used for a Knoevenagel condensation for the synthesis of Ozonil®.
Synthesis route :
Like the other aliphatic aldehydes, Aldehyde C-11 undecylic, can be synthesized by reaction of undecanyl halides (chloride, for example) with dimethyl sulfoxide (DMSO), followed by an alkaline treatment with sodium bicarbonate. Other synthetic routes exist, such as a reduction of Rosenmund from undecylenic acid, using an acid chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

