Photo credits: ScenTree SAS
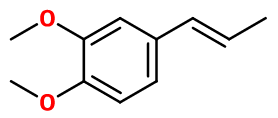
Methyl isoeugenol
Isoeugenol methyl ether ; Isoeugenyl methyl ether ; 1,2-dimethoxy-4-prop-1-enylbenzene ; 3,4-dimethoxy-1,1-propen-1-yl benzene ; Isoeugenol methyl ; Methylisoeugenol ; 4-propenyl veratrole ; 4-prop-1-enyl veratrole ; 1-veratryl-1-propene

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Methyl Iso Eugenol | CL-802 |
Visit website
|
Natural |



|
100 | Eugenia caryophyllus | Clove Oil | Indonesia | 400 Kgs |
General Presentation
-
CAS N° : 93-16-3
-
EINECS number : 202-224-6
-
FEMA number : 2476
-
FLAVIS number : 04.013
-
JECFA number : 1266
-
Appearance : Colorless to pale yellow liquid
-
Density : 1,05
-
Volatility : Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C11H14O2
-
Molecular Weight : 178,23 g/mol
-
Log P : 3,05
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 263°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 113°C
Uses
Uses in perfumery :
Methyl Isoeugenol is used in tuberose and lily accord, to bring a characteristic note, in clove and in spicy flowers accords as carnation.
Year of discovery :
Data not available.
Natural availability :
Methyl Isoeugenol can be found in small quantities in a few essential oils as Star Anise EO, Cinnamon Leaf EO, Mastic Absolute and some varieties of Damask Rose EO. It can be extracted from these oils and can be used on its natural state.
Isomerism :
Methyl Isoeugenol is a position isomer of Methyl Eugenol. Their smell is different : Methyl Isoeugenol is more floral, reminiscent of tuberose, while Methyl Eugenol is more spicy. Both are keeping an earthy and wet facet, more noticeable for Methyl Eugenol. Cis and trans isomers of Methyl Isoeugenol have a very close smell. This explains the general use of a racemic mix of these two isomers in perfumery.
Synthesis precursor :
Methyl Isoeugenol is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Methyl Isoeugenol is obtained in a synthetic way from Isoeugenol, thanks to a methylation reaction. This etherification reaction is done thanks to a Williamson synthesis, consisting in forming a sodium alcoholate, in the presence of pure sodium reacting with Isoeugenol. Then, the Isoeugenolate reacts with methyl chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

