Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 1335-66-6 ; 1423-46-7
-
EINECS number : 215-638-7
-
FEMA number : Donnée indisponible.
-
FLAVIS number : 05.157
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,922
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
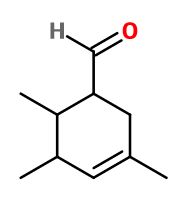
Molecular formula : C10H16O
-
Molecular Weight : 152,24 g/mol
-
Log P : 3,1
-
Fusion Point : -20°C
-
Boiling Point : 203°C
-
Detection Threshold : 5,2547 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 66°C
Uses
Uses in perfumery :
Isocyclocitral® is used in chypre and fougere perfumes, in woody, carnation, geranium, honeysuckle and tomato leaf notes.
Year of discovery :
Data not available.
Natural availability :
Isocyclocitral® is not available in its natural state.
Isomerism :
Isocyclocitral® contains three asymmetric carbons that give various conformational possibilities. As the reagents used for its synthesis are mixtures of diastereoisomers, it is a mixture of isomers that is used in perfumery. Citral is a constitutional isomer of Isocyclocitral®. Nevertheless, its smell is much more hesperidated and less green.
Synthesis precursor :
Isocyclocitral® forms a Schiff base by reaction with Methyl Anthranilate. The resulting compound is quite woody and close to orange blossom.
Synthesis route :
The molecule of Isocyclocitral® can be synthesized by a Diels-Alder reaction between 2-butenal and 2-methylpenta-1,3-diene.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,54 % 0,16 % 3,2 % 3 % 0,76 % 0,76 % 0,76 % 0,76 %1,8 % Cat.5A B C DCat.6 0,76 % 0,76 % 0,76 % 0,76 %1,8 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 6,1 % 6,1 %0,32 % 5,9 % 21 % 21 %12 % 12 %No Restriction Cat.10A BCat.11A BCat.12 21 % 21 %12 % 12 %No Restriction


