Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Citral - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
CITRAL | M_0053264 |
Visit website
|
Naturel | - | - | - | - | - | |
|
|
Citral Ex Lemongrass | 4410001085 |
Visit website
|
Naturel | - | - | - | - | - | |
|
|
Citral Extra | 30035068 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citral FCC | 30035012 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citral FG | 30035059 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citral FG BMBcert™ | 30787698 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citral N | 30035011 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citral N BMBcert™ | 30787697 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 5392-40-5
-
EINECS number : 226-394-6
-
FEMA number : 2303
-
FLAVIS number : 05.020
-
JECFA number : 1225
-
Appearance : Colorless to pale yellow liquid
-
Density : 0,89
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C10H16O
-
Molecular Weight : 152,24 g/mol
-
Log P : 2,9
-
Fusion Point : -20°C
-
Boiling Point : 229°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 98°C
Uses
Uses in perfumery :
Citral is used in citrus notes and especially lemon notes. Can be mixed with D-Limonene for a sweet facet. Brings a fresh facet to ginger and shades the camphorated facet of cardamom.
Year of discovery :
Discovered in 1889.
Natural availability :
Several essential oils are called 'Citral essential oils'. Lemongrass EO can contain up to 85% Citral, Litsea Cubeba EO up to 75% and Verbena up to about 40%. Citral can therefore be obtained naturally from these essential oils.
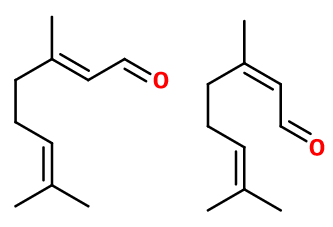
Isomerism :
Citral is actually a mixture of two molecules: Geranial and Neral, with a similar smell. Both are enantiomers, as Citral contains a double bond. Thus, Geranial is the trans (E) isomer and Neral is the cis (Z) isomer of Citral. Both can be separated by adding hydrogen sulfite salts in the mixture. This allows to separate them and convert them back to their original form afterwards. Isocyclocitral® and Camphor are constitutional isomers of Citral. Isocyclocitral has a citrus smell, although greener and earthier.
Synthesis precursor :
Citral is an unsaturated aldehyde, capable of forming many compounds with an organoleptic interest or for other applications. For example, it may be subjected to a condensation of the aldehyde function with another carbonyl function, or be cyclized, or even polymerized. In addition, hydrogenation of Citral leads to Geraniol, Citronellol or 1,3-Dimethyl-1-octanol. It is also at the origin of the synthesis of pseudoionones, by condensation with an active methylene group. These compounds allow to obtain ionones (Alpha-Ionone, Beta-Ionone) or vitamins (vitamin A for example). Finally, Citral forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Synthesis route :
The synthetic production of Citral takes many forms. A copper-catalysed dehydrogenation in vapor phase allows Citral to be obtained from Geraniol or from a mixture of Geraniol and Nerol. Another synthetic route is made from Dehydrolinalool (Linalool containing an alcyne function instead of an alcene) simply by puting it in the presence of a vanadium catalyst such as sodium orthovanadate.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,11 % 0,032 % 0,1 % 0,6 % 0,15 % 0,15 % 0,15 % 0,051 %0,35 % Cat.5A B C DCat.6 0,15 % 0,15 % 0,15 % 0,051 %0,35 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,2 % 0,2 %0,051 % 1,2 % 1,2 % 4,2 %0,051 % 0,051 %No Restriction Cat.10A BCat.11A BCat.12 1,2 % 4,2 %0,051 % 0,051 %No Restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Cardamom seed oil | Elettaria cardamomum (L.) Maton | 8000-66-6 | 0,5 |
| Cassie absolute | Vachellia farnesiana (L.) Willd. | 8023-82-3 | 0,03 |
| Lemon essence oil | Citrus limon (L.) Osbeck | 8008-56-8 | 2,03 |
| Lemon oil, expressed | Citrus limon (L.) Burm. f. | 8008-56-8 | 3,5 |
| Lemon oil, terpeneless | Citrus limon (L.) Osbeck | 68648-39-5 | 40 |
| Citronella oil, Ceylon type | Cymbopogon nardus (L.) Rendle | 8000-29-1 | 0,8 |
| Citronella oil, Java type | Cymbopogon winterianus Jowitt | 8000-29-1 | 0,8 |
| Eucalyptus radiata oil | Eucalyptus radiata Sieber ex DC oil | 92201-64-4 | 1,5 |
| Geranium oil | Pelargonium graveolens l'Hertier ex Aiton | 8000-46-2 | 0,5 |
| Geranium oil African | Pelargonium odoratissimum L'Heritier | 8000-46-2 | 0,5 |
| Ginger oil | Zingiber officinale Rosc. | 8007-08-7 | 2,8 |
| Ginger oleoresin | Zingiber officinale Rosc. | 8007-08-7 | 0,8 |
| Lemongrass oil, West Indian | Cymbopogon citratus (DC) Stapf. | 8007-02-1 | 73 |
| Litsea cubeba oil | Litsea Cubeba(Lour.) Pers. | 68855-99-2 | 69 |
| Mentha citrata oil | Mentha citrata Ehrhart | 68917-15-7 | 0,03 |
| Orange essence oil | Citrus sinensis (L.) Osbeck | 68514-75-0 | 0,15 |
| Orange, sweet, Valencia oil | Citrus sinensis (L.) Osbeck | 8008-57-9 | 0,15 |
| Palmarosa oil | Cymbopogon martinii (Roxb.) Wats | 8014-19-5 | 0,6 |
| Grapefruit oil | Citrus paradisi Macf. | 8016-20-4 | 0,1 |
| Grapefruit oil, terpeneless | Citrus x paradisi Macfad. | 68916-46-1 | 10 |
| Grapefruit oil terpenes | Citrus x paradisi Macfad. | 68917-32-8 | 0,01 |
| Perilla oil | Perilla frutescens (L.) Britton | 68132-21-8 | 0,17 |
| Petitgrain bigarade oil | Citrus aurantium L. spp. Amara Link | 8014-17-3 | 0,65 |
| Petitgrain mandarin oil | Citrus reticulata Blanco | 8014-17-3 | 0,14 |
| Petitgrain lemon oil | Citrus limon (L.) Burm. f. | 8048-51-9 | 23 |
| Rose concrete | Rosa x damascena Mill. | 90106-38-0 | 0,16 |
| Tangerine oil | Citrus reticulata blanco | 8016-85-1 | 0,1 |
| Verbena absolute | Lippia citriodora (L.) Kunth | 8024-12-2 | 25,6 |
| Ylang ylang oil I | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,12 |
| Ylang ylang oil II | Cananga odorata (Lam.) Hook.f.&Thomson forma genuina | 8006-81-3 | 0,09 |
| Ylang ylang oil III | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,02 |
| Ylang, Ylang oil extra | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 0,01 |
| Citrus junos oil | Citrus junos Siebold ex. Tanaka ichangensis × reticulata var. austera (reticulata var. austera) (Rutaceae) | 233683-84-6 | 0,01 |
| Cardamom seed absolute | Elettaria cardamomum (L.) Maton | 96507-91-4 | 0,88 |
| Cardamom seed CO2 extract | Elettaria cardamomum (L.) Maton | 96507-91-4 | 0,88 |
| Clementine oil | Citrus clementina Hort. Ex Tan | 93686-22-7 | 0,15 |
| Murcote oil, expressed | Citrus reticulata spp. murcote, Swingle | 93686-22-7 | 0,1 |
| Tangor oil, expressed | Citrus reticulata x Citrus sinensis | 93686-22-7 | 0,1 |
| Cyperus articulatus oil | Cyperus articulatus L. | 799259-56-6 | 0,7 |
| Kumquat oil, Fortunella margarita | Fortunella (Lour.) Swingle | 938464-05-2 | 10 |
| Meyer lemon oil. cold pressed | Citrus x meyerii | 1370641-98-7 | 5 |
| Petitgrain bigarade absolute | Citrus aurantium L. | 8030-28-2 | 0,2 |
| Orange oil, sweet, terpenes | Citrus sinensis (L.) Osbeck | 68647-72-3; | 0,01 |
| Tangelo oil, expressed | Citrus x tangelo Ingram and Moore | 72869-73-9 | 0,1 |


