Photo credits: ScenTree SAS
Iralia®
N-1-(2,6,6-trimethyl-1-cyclohex-2-enyl)pent-1-en-3-one ; Cyclocitrylidene methyl ethyl ketone ; Iralia total ; Methyl cyclocitrilydene acetone ; Methyl ionone ; N-5-(2,6,6-trimethyl-2-cyclohexen-1-yl)-4-penten-3-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Methylionone 70 | 30235803 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Methylionone 70 BMBcert™ | 30785403 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 1335-46-2
-
EINECS number : 215-635-0
-
FEMA number : 2711
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,931
-
Volatility : Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C14H22O
-
Molecular Weight : 206,33 g/mol
-
Log P : 4,14-4,33
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >110°C (>230°F)
Uses
Uses in perfumery :
Iralia® is used in floral notes of violet, carnation and in spicy tea notes. Often associated with Hedione®. Used in chypre notes for a different facet.
Year of discovery :
Iralia®' trademark has been published and protected by Firmenich SA since 07/11/1966 (brand N°325224)
Natural availability :
Iralia® is not available in its natural state.
Isomerism :
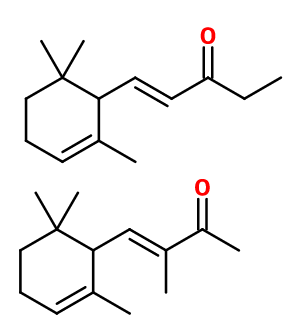
Iralia® is a mixture of molecules consisting mainly of alpha-isomethyl ionone, alpha-methyl ionone and a minority of beta-methyl ionone. All these molecules are isomers.
Synthesis precursor :
Iralia® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Iralia® and methylionones is made from Citral and methyl ethyl ketone (instead of acetone for ionones). This synthesis step gives rise to two molecules called n-Methyl pseudoionone and Isomethyl pseudoionone, both having cis and trans diastereoisomers. The ratio of one molecule relative to the other is favoured by the choice of the reaction catalyst: the more alkaline the catalyst, the more Isomethyl pseudoionone will be favoured. For the cyclization step of Pseudoionone, as for conventional ionones, one isomer will be favoured depending on which acid is used.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 5,4 % 1,6 % 32 % 30 % 7,6 % 7,6 % 7,6 % 7,6 %18 % Cat.5A B C DCat.6 7,6 % 7,6 % 7,6 % 7,6 %18 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 61 % 61 %3,2 % 59 % 100 % 100 %100 % 100 %No Restriction Cat.10A BCat.11A BCat.12 100 % 100 %100 % 100 %No Restriction
-
Restricted ingredients: notes
The above limits apply to Methyl ionone isomers used individually or in combination.
Specified ingredients: notes
Pseudo methyl ionones (CAS numbers 26651-96-7, 72968-25-3, 1117-41-5) should not be used as fragrance ingredient as such. A level of up to 2% of Pseudo methyl ionones as an impurity in Methyl ionones is accepted.


