Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Folione® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 111-12-6
-
EINECS number : 203-836-6
-
FEMA number : 2729
-
FLAVIS number : 09.158
-
JECFA number : 1357
-
Appearance : Colorless liquid
-
Density : 0,92
-
Volatility : Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C9H14O2
-
Molecular Weight : 154,21 g/mol
-
Log P : 2,6
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 219°C
-
Detection Threshold : 25 ppb (0,0000025 %)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 89°C
Uses
Uses in perfumery :
Folione® is used in peach accords, to replace Methyl Octine Carbonate, and for violet leaf notes. Gives a green, cucumber and mushroom effect.
Year of discovery :
1903
Natural availability :
Folione® is not found in nature.
Isomerism :
Folione® is a constitutional isomer of Octahydrocoumarin, even if these two ingredient do not have the same smell at all.
Synthesis precursor :
Folione® is not a precursor to the synthesis of any other molecule used in perfumery.
Synthesis route :
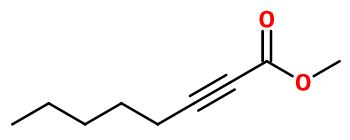
Folione®, or Methyl 2-Octynoate, is synthesized by an esterification reaction involving oct-2-ynoic acid and methanol. This reaction is catalized by the presence of a strong concentrated acid as sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,0085 % 0,0025 % 0,051 % 0,047 % 0,012 % 0,012 % 0,012 % 0,012 %0,028 % Cat.5A B C DCat.6 0,012 % 0,012 % 0,012 % 0,012 %0,028 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,096 % 0,096 %0,005 % 0,092 % 0,33 % 0,33 %0,18 % 0,18 %No Restriction Cat.10A BCat.11A BCat.12 0,33 % 0,33 %0,18 % 0,18 %No Restriction
-
Restricted ingredients: notes
When used in the same fragrance compound within a specific QRA category, the sum total of Methyl heptine carbonate (MHC, CAS number 111-12-6) and Methyl octine carbonate (MOC, CAS number 111-80-8) contributions must not exceed the maximum permitted level for MHC. At the same time, the contribution from MOC should always respect the maximum levels permitted in the respective categories as listed in the Standard for MOC.


