Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Eugenyl Acetate | CL-901 |
Visit website
|
Natural |



|
100 | Eugenia caryophyllus | Clove Oil | Indonesia | 400 Kgs |
|
|
EUGENYL ACETATE | M_0053471 |
Visit website
|
Synthétique | - | - | - | - | - |
General Presentation
-
CAS N° : 93-28-7
-
EINECS number : 202-235-6
-
FEMA number : 2469
-
FLAVIS number : 09.020
-
JECFA number : 1531
-
Appearance : Colorless liquid that solidifies at room temperature
-
Density : 1,079
-
Volatility : Heart/Base
-
Price Range : €€€
Physico-chemical properties
-
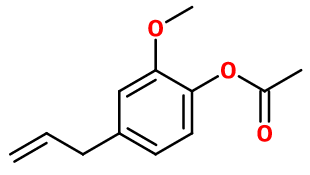
Molecular formula : C12H14O3
-
Molecular Weight : 206,24 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : 25°C
-
Boiling Point : 284°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 110°C
Uses
Uses in perfumery :
Eugenyl acetate is used in clove notes to deepen a floral character, or in white floral notes such as jasmine to affirm its spicy facet. Very good for jasmine tea notes.
Year of discovery :
Data not available.
Natural availability :
Eugenyl acetate is present in a relatively small amount in Ceylon Cinnamon EO (and other origins), Cinnamon Leaf EO, Clove Bud EO, Clove Leaf EO, in Bay St-Thomas EO and Laurel Bay EO among others. It can be extracted in its natural state from all these essential oils.
Isomerism :
Aldehyde C-16 and Aldehyde C-20 are constitutional isomers of Eugenyl acetate. However, their smell is quite different, as they are fruity rather than spicy.
Synthesis precursor :
Eugenyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Eugenyl acetate is synthesized by an esterification reaction between acetic acid and Eugenol. The reaction is catalysed by the presence of a strong acid in a small quantity, such as concentrated sulfuric acid. For a better yield, the reaction may be done with acetic anhydride or chloroacetic acid instead of acetic acid.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

