Photo credits: ScenTree SAS
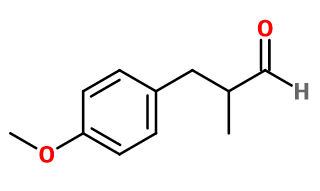
Canthoxal®
Anisyl Propanal ; 3-(4-methoxyphenyl)-2-methylpropanal ; Foliaver® ; Fennaldehyde ; Cantonal ; Canthorg ; Floral anise ; 2-methoxy-3-(para-methoxyphenyl) propanal ; 4-methoxy-alpha-methyl benzene propanal ; Para-methoxy-alpha-methyl hydrocinnamaldehyde ; Methoxyhydratropaldehyde ; Methoxyphenal ; 3-(para- methoxyphenyl)-2-methyl propionaldehyde ; 3-(4- methoxyphenyl)-2-methylpropanal ; Alpha- methyl-4-methoxybenzene propanal

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Canthoxal® - 30gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
SCHIFF CANTHOXAL | 85709 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 5462-06-6
-
EINECS number : 226-749-5
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 1,043
-
Volatility : Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C11H14O2
-
Molecular Weight : 178,23 g/mol
-
Log P : 2,5
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 108°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 94°C
Uses
Uses in perfumery :
Canthoxal® is used to create juicy fruits accords (melon, pear...) and floral notes (mimosa, brooms, lilies...). Can be used both as a booster for a cologne head or as a fresh note for oriental perfumes.
Year of discovery :
1951
Natural availability :
Canthoxal® is not available in its natural state.
Isomerism :
Canthoxal® has an asymmetrical carbon that gives rise to two enantiomers. However, it is the racemic mixture of these two isomers that is used in perfumery. Methyl Eugenol is a constitutional isomer of Canthoxal®. Its smell is however much more spicy and earthy.
Synthesis precursor :
Canthoxal® forms a Schiff base by reaction with Methyl Anthranilate or Indole for example.
Synthesis route :
Canthoxal® is synthesized by a condensation reaction of Anisic Aldehyde with propanal, followed by a hydrogenation of the intermediate product.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,11 % 0,14 % 0,75 % 2,5 % 0,64 % 0,64 % 0,64 % 0,21 %0,11 % Cat.5A B C DCat.6 0,64 % 0,64 % 0,64 % 0,21 %0,11 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,86 % 0,86 %0,21 % 2,7 % 0,75 % 4,1 %0,21 % 0,21 %No restriction Cat.10A BCat.11A BCat.12 0,75 % 4,1 %0,21 % 0,21 %No restriction


