Photo credits: ScenTree SAS
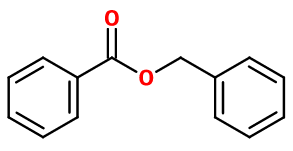
Benzyl benzoate
Phenylmethyl benzoate ; Benzoate de phénylméthyle ; Ascabin ; Ascabiol ; Benzyl benzoate ; Antiscabiosum ; Benylate ; Benzyl phenyl formate ; Benzylbenzoate ; Benzylets ; Colebenz ; Novoscabin ; Peruscabin ; Phenyl methyl benzoate ; Scabanca ; Scobenol ; Vanzoate ; Venzoate

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Benzoate de Benzyle 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 120-51-4
-
EINECS number : 204-402-9
-
FEMA number : 2138
-
FLAVIS number : 09.727
-
JECFA number : 24
-
Appearance : Colorless liquid
-
Density : 1,1
-
Volatility : Base
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C14H12O2
-
Molecular Weight : 212,25 g/mol
-
Log P : 3,97
-
Fusion Point : 21°C
-
Boiling Point : 323°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 148°C
Uses
Uses in perfumery :
Benzyl Benzoate is usually used for diluting raw materials as resinoids or Galaxolide®, to reduce its vicosity for use. It is also used in compositions as a fixative and a heavy floral notes modifier, in jasmine and other white flowers accords.
Year of discovery :
Data not available.
Natural availability :
Benzyl Benzoate is the major component of Peru Balsam Resinoid. It is also found in Tuberose Absolute, Grandiflorum Jasmine Absolute, Narcissus Absolute, Frangipani Absolute…
Isomerism :
Benzyl Benzoate does not have any isomer used in perfumery.
Synthesis precursor :
Benzyl Benzoate is not used in the synthesis of another compound of olfactive interest.
Synthesis route :
Benzyl Benzoate can be synthesized in three ways. The first method is a transesterification of technical Methyl Benzoate, with Benzyl Alcohol. A second synthesis way makes benzoyl chloride react with sodium benzoate. A last method is the Tishchenko reaction from Benzaldehyde, with sodium or aluminium benzylate.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 1,7 % 1,4 % 0,41 % 4,8 % 4,3 % 0,21 % 0,83 % 0,07 %0,41 % Cat.5A B C DCat.6 4,3 % 0,21 % 0,83 % 0,07 %0,41 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,41 % 0,41 %0,07 % 1,9 % 1,9 % 12 %0,07 % 0,07 %No Restriction Cat.10A BCat.11A BCat.12 1,9 % 12 %0,07 % 0,07 %No Restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Balsam absolute, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 32,5 |
| Balsam oil, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 59,17 |
| Balsam resinoid, Peru | Myroxylon balsamum (L.) Harms var. pereirae (Royle) Harms | 8007-00-9 | 14,16 |
| Benzoin infusion, Sumatra | Styrax paralleloneurum Perkins | 0,018 | |
| Rosewood oil | Aniba rosaeodora (Ducke) var amazonica | 8015-77-8 | 1,2 |
| Cananga oil | Cananga odorata (Lam.) Hook. f. & Thomson (forma macrophylla Steenis) | 68606-83-7 | 4,2 |
| Cinnamon bark oil, Laos | Cinnamomum loureiroi Nees | 97659-68-2 | 0,01 |
| Cinnamon bark CO2 extract | Cinnamomum verum J. Presl syn. C. zeylanicum Blume | 8015-91-6 | 1,8 |
| Cinnamon bark oil | Cinnamomum zeylanicum Blume | 8015-91-6 | 0,6 |
| Cinnamon leaf oil | Cinnamomum zeylanicum Blume | 8015-91-6 | 3,5 |
| Cassia bark oil | Cinnamomum cassia [L.] J. Presl syn. C. aromaticum Nees | 8007-80-5 | 0,08 |
| Cassia bark oleoresin | Cinnamomum cassia [L.] J. Presl syn. C. aromaticum Nees | 8007-80-5 | 0,06 |
| Cassia oil | Cinnamomum cassia [L.] J. Presl syn. C. aromaticum Nees | 8007-80-5 | 0,1 |
| Cassie absolute | Vachellia farnesiana (L.) Willd. | 8023-82-3 | 0,05 |
| Champaca absolute | Michelia champaca L. | 8006-76-6 | 0,53 |
| Jasmine absolute | Jasmium grandifloum L. | 8022-96-6 | 10,07 |
| Jasmine CO2 extract | Jasmium grandifloum L. | 8022-96-6 | 5 |
| Jasmine concrete | Jasminum grandiflorum L. | 8022-96-6 | 10,07 |
| Mimosa absolute | Acacia decurrens (Wendl.f.) Willd. | 8031-03-6 | 0,12 |
| Narcissus poeticus absolute | Narcissus poeticus L. | 68917-12-4 | 8,9 |
| Carnation absolute | Dianthus caryophyllus L. | 8021-43-0 | 13 |
| Rose oil | Rosa x damascena Mill. | 8007-01-0 | 0,07 |
| Rose absolute | Rosa x damascena Mill. | 90106-38-0 | 0,02 |
| Styrax oil | Liquidambar styraciflua L. | 8046-19-3 | 0,02 |
| Tuberose absolute | Poliantes tuberosa L. | 8024-05-3 | 5,5 |
| Tuberose concrete | Poliantes tuberosa L. | 8024-05-3 | 0,72 |
| Ylang ylang oil I | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 5,86 |
| Ylang ylang oil II | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 7,81 |
| Ylang ylang oil III | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 6,23 |
| Ylang, Ylang oil complete | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 6,32 |
| Ylang, Ylang oil extra | Cananga odorata (Lam.) Hook. f. &Thomson oil (forma genuine Steenis) | 8006-81-3 | 5,73 |
| Clove bud absolute | Syzygium aromaticum (L.) Merr. & L.M.Perry | 616-772-2 | 0,07 |
| Hyacinth absolute | Hyacinthus orientalis L. | 8023-94-7 | 6 |
| Jasmine officinale absolute | Jasminum officinale L. | 8024-43-9 | 10 |
| Jasmine sambac absolute | Jasminum sambac (L.) Aiton | 103798-23-6 | 0,75 |
| Jasmine sambac CO2 extract | Jasminum sambac | 103798-23-6 | 0,75 |
| Ylang-ylang absolute | Cananga odorata (Lam.) Hook.f.&Thomson forma genuina | 7 | |
| Benzoin resinoid, Siam | Styrax tonkinensis (Pierre) Craib ex Hartwick | 0,25 | |
| Benzoin resinoid, Sumatra | Styrax benzoin Dryand. | 0,04 | |


