Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 628-63-7
-
EINECS number : 211-047-3
-
FEMA number : --
-
FLAVIS number : 09.021
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,876
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
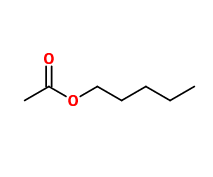
Molecular formula : C7H14O2
-
Molecular Weight : 130,19 g/mol
-
Log P : 2,29
-
Fusion Point : -71°C
-
Boiling Point : 142°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 41°C
Uses
Uses in perfumery :
Amyl acetate is used in majority in fruity accords, associated with white flowers (as jasmine) and aqueous compounds, to boost the top note, bringing a summer fruit aspect.
Year of discovery :
Data not available.
Natural availability :
Amyl acetate is found in the odorous principle of many fruits as apple and peach, but its synthetic version is more used in perfumes.
Isomerism :
Amyl acetate is a positional isomer of Isoamyl acetate. The smell of Isoamyl acetate is known as more reminiscent of banana fruit, when Amyl acetate is more associated to pear fruit. Amyl compounds frequently have a mushroom smell.
Synthesis precursor :
Amyl acetate is not a precursor to the synthesis of another compound of olfactive interest.
Synthesis route :
Amyl acetate is synthesized by an esterification reaction between acetic acid and pentanol (or amyl alcohol). This synthesis can be optimized to get a better yield, replacing acetic acid by acetic anhydride or chloroacetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

