Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate d'Isoamyle - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
ISOAMYL ACETATE | M_0050642 |
Visit website
|
Naturel | - | - | - | - | - | |
|
|
3-METHYLBUTYL ACETATE | 441I001000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | UK | - |
General Presentation
-
CAS N° : 123-92-2
-
EINECS number : 204-662-3
-
FEMA number : 2055
-
FLAVIS number : 09.024
-
JECFA number : 43
-
Appearance : Colorless liquid
-
Density : 0,876
-
Volatility : Head
-
Price Range : €
Physico-chemical properties
-
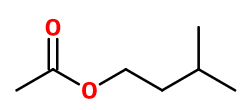
Molecular formula : C7H14O2
-
Molecular Weight : 130,19 g/mol
-
Log P : 2,7
-
Fusion Point : -78°C
-
Boiling Point : 142°C
-
Detection Threshold : 2 à 43 ppb (0,0000043%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 25°C
Uses
Uses in perfumery :
Isoamyl acetate is used in banana notes. Enhances the head of a perfume, and gives a fruity-banana or pear nuance, especially in jasmine notes. However, it can make an accord become very artificial in the case of an overdose.
Year of discovery :
Data not available.
Natural availability :
Isoamyl acetate can be extracted from Roman Chamomile EO, although it is present in very small quantities.
Isomerism :
Isoamyl acetate does not have any isomer used in perfumery.
Synthesis precursor :
Isoamyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Isoamyl acetate is synthesized by an esterification reaction between acetic acid or acetic anhydride and Isoamyl alcohol, in the presence of an acid catalyst.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

