Photo credits: ScenTree SAS
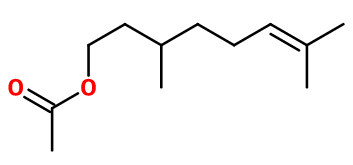
DL-citronellyl acetate
Citronellyl acetate ; 3,7-dimethyloct-6-enyl acetate ; acetate de 3,7-dimethyloct-6-enyl ; Citronellyl ethanoate ; Acetic acid citronellyl ester ; Citronellol acetate ; 3,7-dimethyloct-6-enol acetate ; 3,7-dimethyloct-6-enyl ethanoate ; 3,7-dimethyloct-6-enol ethanoate

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Acetate de Citronellyle - 30 Gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
Citronellyl Acetate | 30035076 |
Visit website
|
Molecule | - | - | - | - | - | |
|
|
Citronellyl Acetate BMBcert™ | 30786719 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 150-84-5
-
EINECS number : 205-775-0
-
FEMA number : 2311
-
FLAVIS number : 09.012
-
JECFA number : 57
-
Appearance : Colorless liquid
-
Density : 0,891
-
Volatility : Head/Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C12H22O2
-
Molecular Weight : 198,31 g/mol
-
Log P : 4,22
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 240°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 93°C
Uses
Uses in perfumery :
Citronellyl acetate is used for a fruity note in rose, lily of the valley and lavender accords for example.
Year of discovery :
Data not available.
Natural availability :
Citronellyl acetate is naturally present in Lemongrass EO, Geranium EO and Rose de Mai Absolute, and therefore is extractable in order to obtain natural Citronellyl acetate.
Isomerism :
The asymmetric carbon of Citronellol gives it two different smells if its enantiomers are separated: the (R)-(+)-Citronellyl acetate is fruity and rosy, while the (S)-(-)-Citronellyl acetate is more aldehydic, dirty and lemony. In perfumery, those two enantiomers can be used separately. In most cases, a mixture of the two is used. Menthanyl acetate, Verdox® and Vertenex® are constitutional isomers of Citronellyl acetate. However, Menthanyl acetate is much more reminiscent of Bergamot EO, and Verdox® and Vertenex® are woodier.
Synthesis precursor :
Citronellyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Citronellyl acetate can be synthesized by an esterification reaction between Citronellol and acetic acid or acetic anhydride, in an acid medium. It can also be synthesized from 3,7-dimethylocta-1,6-diene, obtained naturally by pyrolysis of alpha-Pinene. This synthesis is made in three steps: a Markovnikov addition reaction using hydrochloric acid, a Kharasch reaction, also called anti-Markovnikov reaction, with hydrobromic acid, followed by an acetolysis reaction using sodium ethanoate.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,49 % 0,15 % 2,0 % 2,7 % 0,70 % 0,70 % 0,70 % 0,23 %0,82 % Cat.5A B C DCat.6 0,70 % 0,70 % 0,70 % 0,23 %0,82 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 2,4 % 2,4 %0,23 % 5,4 % 0,41 % 16 %0,23 % 0,23 %No restriction Cat.10A BCat.11A BCat.12 0,41 % 16 %0,23 % 0,23 %No restriction
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Juniper berry oil | Juniperus communis L. | 8002-68-4 | 0,19 |
| Schinus molle oil | Schinus areira L. | 68917-52-2 | 0,14 |
| Schinus terebenthifolius CO2 extract | Schinus terebenthifolius Raddi | 949495-68-5 | 0,25 |
| Basil oil, chemotype linalool | Ocimum basilicum L. | 8015-73-4 | 0,1 |
| Lemon oil, terpeneless | Citrus limon (L.) Osbeck | 68648-39-5 | 1,72 |
| Citronella oil, Ceylon type | Cymbopogon nardus (L.) Rendle | 8000-29-1 | 0,9 |
| Citronella oil, Java type | Cymbopogon winterianus Jowitt | 8000-29-1 | 3 |
| Eucalyptus citriodora oil | Corymbia citriodora (Hook.) K.D. Hill & L.A. Johnson | 85203-56-1 | 1,81 |
| Geranium absolute | Pelargonium graveolens l'Hertier ex Aiton | 8000-46-2 | 0,41 |
| Geranium oil | Pelargonium graveolens l'Hertier ex Aiton | 8000-46-2 | 0,5 |
| Geranium oil African | Pelargonium odoratissimum L'Heritier | 8000-46-2 | 0,8 |
| Ginger CO2 extract | Zingiber officinalle Roscoe | 8007-08-7 | 0,17 |
| Lemongrass oil, East Indian | Cymbopogon flexuosus (Nees ex Steudel) Will. Watson | 8007-02-1 | 0,23 |
| Balm oil | Melissa officinallis L. | 8014-71-9 | 1,35 |
| Nutmeg oil | Myristica fragrans Houtt. | 8008-45-5 | 0,13 |
| Grapefruit oil, terpeneless | Citrus x paradisi Macfad. | 68916-46-1 | 0,31 |
| Petitgrain lemon oil | Citrus limon (L.) Burm. f. | 8048-51-9 | 0,2 |
| Rose oil | Rosa x damascena Mill. | 8007-01-0 | 0,55 |
| Rose water stronger | Rosa x centifolia L. | 8007-01-0 | 0,02 |
| Rose absolute | Rosa x damascena Mill. | 90106-38-0 | 0,13 |
| Rose concrete | Rosa x damascena Mill. | 90106-38-0 | 0,03 |
| Abies alba cone oil | Abies alba Mill. | 8021-27-0 | 0,05 |
| Bucchu absolute, betulina | Agathosma betulina (P.J.Bergius) Pillans | 84649-93-4 | 0,15 |
| Citrus hystrix extract | Citrus hystrix DC | 91771-50-5 | 0,4 |
| Tagetes minuta oil | Tagetes minuta L. | 616-989-2 | 0,43 |


