Vertenex®
PTBCH acetate (ParaTertButylCycloHexanol) ; (4-tert-butylcyclohexyl) acetate ; Para-tert-butyl cyclohexyl acetate ; 4-tert-butyl cyclohexyl acetate ; 4-tert-butylcyclohexanol acetate ; Lorysia ; Oryclon ; 4-tert-pentylcyclohexanyl acetate ; Rodonex ; Woody acetate
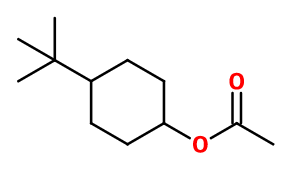
Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Naturality | Purity | Latin name | Treated part | Geographical origin | Certifications | Comments | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Vertenex - 30 Gr | - | - | - | - | - | - | more | - |
General Presentation
-
CAS N° : : 32210-23-4
-
EINECS number : 250-954-9
-
FEMA number : Donnée indisponible.
-
Density : 0,937
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €
-
Appearance : Colorless liquid
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 230°C (à 33 hPa)
-
Detection Threshold : Donnée indisponible.
-
Molecular formula : C12H22O2
-
Log P : 4,23
-
Molecular Weight : 198,31 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 100°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
In comparision to Vertenex®, Verdox® has a more fruity note, reminiscent of apple, before also being woody.
Stability :
Stable in perfumes and diverse functional bases
Uses in perfumery :
Vertenex® is used in functional fragrance for its stability and substantivity. Used in fougere, marine, fruity notes. Making the link between a fruity or floral note with a woody note.
Year of discovery :
1948
Isomerism :
The trans isomer of Vertenex® has a very woody smell, while the cis isomer has a more fruity and floral smell. One of the positional isomers of Vertenex® is Verdox®, with a less woody smell. Citronellyl acetate and Menthanyl acetate are constitutional isomers of Verdox®. Their smell is distinctly different. Although also fruity, they do not have a woody note.
Synthesis precursor :
Vertenex® is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Vertenex® is not available in its natural state.
Synthesis route :
Vertenex® is synthesized by a first catalytic hydrogenation step of tertbutyl-phenol, at high temperature and pressure. Acetylation of the resulting compound is made by reaction with acetic anhydride and in the presence of an acid catalyst. For the hydrogenation phase, if nickel is used as a catalyst, the trans isomer is predominantly obtained. On the other hand, if the catalysis is made with rhodium, it is the cis isomer which is found in majority at the end of the reaction.
Regulations & IFRA
This ingredient is not restricted

