Photo credits: ScenTree SAS
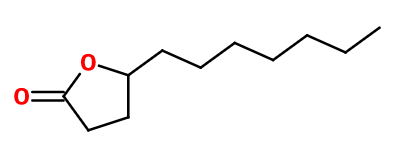
Aldehyde C-14
γ-Undecalactone ; 5-heptyloxolan-2-one ; Abriceine ; Dihydro-5-heptyl-2-(3H)-furanone ; Undecylene methyl lactone ; Peach aldehyde ; Peach lactone ; Peach pure ; Pèche pure ; 4-undecanolide ; Undecalactone gamma cœur

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Aldéhyde C14 Gamma Undecalactone - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 104-67-6
-
EINECS number : 203-225-4
-
FEMA number : 3091
-
FLAVIS number : 10.002
-
JECFA number : 233
-
Appearance : Colorless liquid
-
Density : 0,949
-
Volatility : Base
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C11H20O2
-
Molecular Weight : 184,27 g/mol
-
Log P : 4
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 165°C
-
Detection Threshold : 60 ppb (0,000006%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 113°C
Uses
Uses in perfumery :
Aldehyde C-14 is used in fruity, floral, exotic notes. To be combined with Aldehyde C18 (gamma-nonalactone). Component used in a reproduction of a peach note.
Year of discovery :
Discovered in 1905.
Natural availability :
Aldehyde C-14 is not available in its natural state.
Isomerism :
Aldehyde C-14 has two isomers (R) and (S) as it contains an asymmetric carbon. The (R) enantiomer has a peachy smell while the (S) enantiomer has a more aldehydic smell. The racemic mixture of the two enantiomers is the compound that is used in perfumery. Citronellyl Formate is a constitutional isomer of Aldehyde C-14. Its smell is more reminiscent of pear and rose than peach.
Synthesis precursor :
Aldehyde C-14 is not a precursor for the synthesis of another compound of olfactory interest.
Synthesis route :
Aldehyde C-14 is actually a lactone, in other words, a cyclic ester. Its synthesis is carried out by a free-radical addition of 1-octanol to acrylic acid. It can also be prepared by a reaction between 10-undecylenic aldehyde and sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

