Gamma-nonalactone
Aldehyde C18 ; Coconut Aldehyde ; 5-pentyloxolan-2-one ; Prunolide ; Abricolin ; Gamma-amyl butyrolactone ; 4-amyl-4-hydroxybutyric acid lactone ; Amylbutyrolactone ; Apricolin ; Coconut aldehyde ; Dihydro-5-pentyl-2(3H)-furanone ; 4-nonalactone ; 1,4-nonalolide ; 1,4-nonanolide ; Gamma-nonanolide ; Gamma-nonyl lactone ; Gamma- pelargolactone ; 4-pentyl butanolide ; 5-pentyl-dihydro-furan-2-one ; 5- pentyloxolan-2-one
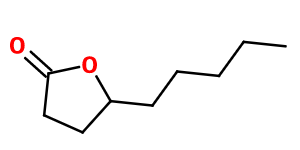
Photo credits: ScenTree SAS
General Presentation
-
CAS N° : : 104-61-0
-
EINECS number : 203-219-1
-
FEMA number : 2781
-
Density : 0,964
-
Optical rotation : Lorem Ipsum
-
Allergens : This ingredient does not contain any allergen.
-
Refractive Index @20°C : Lorem Ipsum
-
Volatility : Heart
-
Price Range : €€
-
Appearance : Colorless liquid
-
FLAVIS number : 10.001
-
JECFA number : 229
Information on synthetic ingredients
-
Acid Value : Lorem Ipsum
-
Boiling Point : 243°C
-
Detection Threshold : 7 ppb (0,0000007%)
-
Molecular formula : C9H16O2
-
Log P : 2,5
-
Molecular Weight : 156,22 g/mol
-
Fusion Point : Donnée indisponible.
-
Flash Point : 126°C
-
Vapor pressure : Lorem Ipsum
Uses
Other comments :
Among the different lactones used in perfumery, Gamma-Nonalactone is an equilibrium between a coconut and a peach note. In that way, it is close to Nectaryl® and Lactone of cis-Jasmone.
Stability :
Lactones tend to polymerize through time, making them more viscous and leading to a phase shift in alcohol.
Uses in perfumery :
Gamma-Nonalactone contributes to an exotic, fruity-peach, milky and gourmet facet, in adequation with fruity notes in particular.
Year of discovery :
Data not available.
Isomerism :
Gamma-Nonalactone, like other lactones, has an asymmetric carbon. The smell of the two enantiomers of this molecule is similar. The racemic mixture is most often used in perfumery. Isoamyl Butyrate and Allyl Caproate are constitutional isomers of gamma-Nonalactone, but they have a very different butyric and cheesy smell.
Synthesis precursor :
Gamma-Nonalactone is not a precursor to the synthesis of another compound of olfactory interest.
Natural availability :
Gamma-Nonalactone is present in several foods and in the Eschweilera coriacea flower, from Central America. However, it is synthetic gamma-Nonalactone which is most often used in perfumery.
Synthesis route :
Gamma-Nonalactone is a cyclic lactone synthesized in the same way as other lactones. The reaction between acrylic acid and hexanol, in the presence of a sulfate or an alkaline phosphate, allows to synthesize this molecule. An intramolecular esterification of 4-hydroxynonanoic acid, catalysed by a strong acid such as concentrated sulfuric acid, also allows to obtain this compound. Finally, biochemical synthesis pathways are being studied by producing companies.
Regulations & IFRA
This ingredient is not restricted

