Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
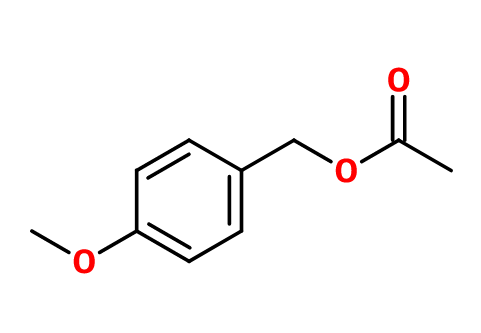
CAS N° : 104-21-2
-
EINECS number : 203-185-8
-
FEMA number : 2098
-
FLAVIS number : 09.019
-
JECFA number : 873
-
Appearance : Liquid
-
Density : 1,116
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C10H12O3
-
Molecular Weight : 180,2 g/mol
-
Log P : 1,9
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 270°C
-
Detection Threshold : 47,257 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 118°C
Uses
Uses in perfumery :
Anisyl acetate is widely used for perfuming cosmetics, for white floral and red fruity notes, for almond, cherry and mimosa notes.
Year of discovery :
Data not available.
Natural availability :
Anisyl acetate is present in several red fruits and in sweet acacia, from which it can be extracted in its natural state. However, synthetic Anisyl acetate remains the most used in perfumery.
Isomerism :
Anisyl acetate as we use it in perfumery is ''para-Anisyl acetate ''. This means that its ether group is opposed to the carboxylic group, with respect to its benzene ring. For example, ortho-Anisyl acetate, whose ether group is directly connected to the carboxylic group, is spicier and drier than para-Anisyl acetate.
Synthesis precursor :
Anisyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Anisyl acetate can be synthesized from different reagents, but its synthesis will always end up by an esterification reaction of the Anisyl Alcohol with acetic acid or acetic anhydride, in an acid medium.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

