Photo credits: ScenTree SAS
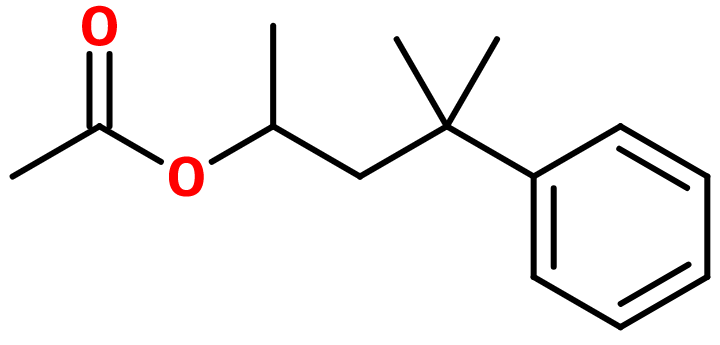
Woodinyl acetate
1,3-dimethyl-3-phenyl butyl acetate ; Isobutyl phenylethyl carbinyl acetate ; Corps 53 ; Rhubarb body ; 4-methyl-4-phenyl-2-pentyl acetate ; 4-methyl-4-phenylpentan-2-yl acetate ; Vetikol acetate ; Vetikyle acetate ; Veticol acetate ; Veticyle acetate ; Corps rhubarb ; Vetac

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 68083-58-9
-
EINECS number : 268-407-8
-
FEMA number : --
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,98
-
Volatility : Head/Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C14H20O2
-
Molecular Weight : 220,31 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 270°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 115°C
Uses
Uses in perfumery :
This molecule is used in floral notes as gardenia, in reconstitutions of rhubarb and grapefruit, in woody notes as vetiver and sandalwood. It brings a milky and warm nuance in eaux fraiches for example.
Year of discovery :
Data not available.
Natural availability :
Woodinyl acetate is not found in nature.
Isomerism :
In its molecular structure, Woodinyl acetate has one asymetric carbon, giving birth to two enantiomers. These are not used separately in perfumery. Woodinyl acetate also is a constitutional isomer of Dimethylbenzylcarbinyl Butyrate, whose molecular structure is similar. The latter is nevertheless more reminiscent of dry fruits, while Woodinyl acetate is of rhubarb.
Synthesis precursor :
Woodinyl acetate is not used for the synthesis of another compound used in perfumery.
Synthesis route :
Woodinyl acetate is prepared with an esterification reaction. This reaction involves 4-methyl-4-phenylpentanol and acetic acid. It is catalyzed by the use of a low quantity of one strong acid as sulfuric acid. This reaction yield can be improved thanks to the use of chloroacetic acid or acetic anhydride instead of acetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

