Photo credits: ScenTree SAS
Verdox®
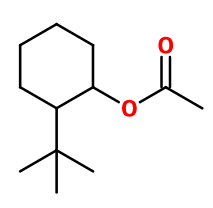
OTBCH acetate (OrthoTertButylCycloHexanol) ; (2-tert-butylcyclohexyl) acetate ; 1-acetoxy-2-tert-butylcyclohexane ; Agrumex HC ; 2-tert-butyl cyclohexanol acetate ; Ortho-tert-butyl cyclohexyl acetate ; 2-(1,1- dimethylethyl)cyclohexyl acetate ; Green acetate ; Grumex ; Menthonate ; Ommelione supra ; Ortholate ; Polarvert ; Pommelione supra ; Ylanat ortho

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Verdox® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 88-41-5
-
EINECS number : 201-828-7
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,942
-
Volatility : Heart
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C12H22O2
-
Molecular Weight : 198,31 g/mol
-
Log P : 4,23
-
Fusion Point : 27°C
-
Boiling Point : 232°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 91°C
Uses
Uses in perfumery :
Verdox® is used in woody, coniferous, apple and fruity tea notes. Sometimes used as a Beta-Damascenone® replacer. Used for its stability in shampoos and shower gels.
Year of discovery :
1956
Natural availability :
Verdox® is not available in its natural state.
Isomerism :
Verdox® is a mixture of isomers, of which the cis isomer is present in majority, representing 60 to 95% of the mixture. One of the positional isomers of Verdox® is Vertenex®, with a woodier smell. Citronellyl acetate and Menthanyl acetate are constitutional isomers of Verdox®. Their smell is distinctly different, and although they also are fruity, they do not have a woody note.
Synthesis precursor :
Verdox® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Verdox® is synthesized from 2-tert-butylcyclohexanol (obtained from 2-tert-butylphenol by a catalytic hydrogenation) by an esterification reaction with acetic anhydride, catalysed by a strong concentrated acid such as sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

