Photo credits: ScenTree SAS
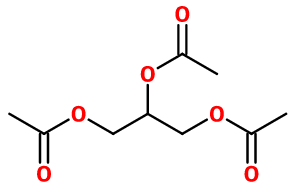
Triacetin
1,3-diacetyloxypropan-2-yl acetate ; 2-acetyloxy-1-(acetyloxymethyl)ethyl acetate ; Captex 500 ; Enzactin ; Fungacetin ; Glycerin triacetate ; Glycerol triacetate ; Glyceryl triacetate ; 1,2,3-propane triol triacetate ; 1,2,3-propanetriol triacetate ; Triacetine ; Triacetylglycerol ; Vanay

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 102-76-1
-
EINECS number : 203-051-9
-
FEMA number : 2007
-
FLAVIS number : Donnée indisponible.
-
JECFA number : 920
-
Appearance : Colorless liquid
-
Density : 1,16
-
Volatility : NON TROUVE_N/A
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C9H14O6
-
Molecular Weight : 218,2 g/mol
-
Log P : 0,25
-
Fusion Point : 3°C
-
Boiling Point : 258°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 138°C
Uses
Uses in perfumery :
Triacetin is a solvent sometimes found in some perfume concentrates.
For stability reasons, this solvent is suitable for use in shower gel base, cream base and dishwashing liquid base.
However, this solvent should not be used in candle base concentrates of any type.
Year of discovery :
Data not available.
Natural availability :
Triacetin is present in the fragrant principle of papaya, but is not extracted from this fruit and is only synthesized for its use in perfumery.
Isomerism :
Triacetin does not have any isomer used in perfumery.
Synthesis precursor :
Triacetin does not intervene in the synthesis of a perfume compound.
Synthesis route :
Triacetin can usually be made from glycerol and acetic acid. This esterification reaction is carried out in the presence of an acid catalyst such as concentrated sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

