Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
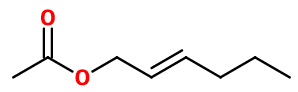
TRANS-2-HEXENYL ACETATE | 2311119510 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | Romania | - |
General Presentation
-
CAS N° : 2497-18-9
-
EINECS number : 219-680-7
-
FEMA number : 2564
-
FLAVIS number : 09.394
-
JECFA number : 1355
-
Appearance : Colorless liquid
-
Density : 0,897
-
Volatility : Head/Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C8H14O2
-
Molecular Weight : 142,2 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 166°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 59°C
Uses
Uses in perfumery :
Trans-2-Hexenyl acetate is mainly used in fruity and green apple or pear notes, among others, to combine a juicy effect and make the link between a fruity and a green facet.
Year of discovery :
Data not available.
Natural availability :
Trans-2-Hexenyl acetate is found in the fragrant principle of certain fruits such as apple, banana or mango, but also in trace amounts in some essential oils such as Peppermint EO. It is therefore its synthetic version that is most used in perfumery.
Isomerism :
Trans-2-hexenyl acetate is a diastereoisomer of cis-2-Hexenyl acetate, much less used in perfumery. On the other hand, cis-3-Hexenyl acetate, the position isomer of trans-2-Hexenyl acetate, has a much greener smell, reminiscent of cut grass, and with substantially equivalent fruity accents.
Synthesis precursor :
Trans-2-Hexenyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Trans-2-Hexenyl acetate can be synthesized by an esterification reaction, by reacting trans-2-Hexenol with acetic acid, in the presence of an acid catalyst such as concentrated sulfuric acid, in very small quantity in the reaction medium. The efficiency of this reaction can be improved by using acetic anhydride or chloroacetic acid instead of acetic acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

