Photo credits: ScenTree SAS
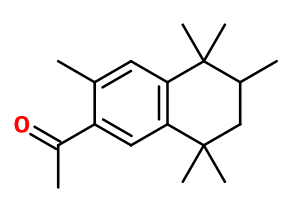
Tonalide®
Ganolide® ; Tetralide® ; 1-(3,5,5,6,8,8-hexamethyl-6,7-dihydronaphthalen-2-yl)ethanone ; Acetyl hexamethyl tetralin ; 6-acetyl-1,1,2,4,4,7-hexamethyl tetralin ; AHMT ; Fixolide ; Muscofix ; Musk tetralin ; 1-(5,6,7,8- tetrahydro-3,5,5,6,8,8-hexamethyl-2-naphthyl)ethan-1-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Tonalide - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 1506-02-1
-
EINECS number : 216-133-4
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : White solid
-
Density : 1,005
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C18H26O
-
Molecular Weight : 258,4 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : 55°C
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >100°C (>212°F)
Uses
Uses in perfumery :
Tonalide® is used in men's fragrances, in combination with other musks. Good fixator and substantivity.
Used in all types of fragrances, especially in body care. Its earthy note can stand out if this raw material is overdosed.
Year of discovery :
Discovered in 1954.
Natural availability :
Tonalide® is not available in its natural state.
Isomerism :
Galaxolide® is another musk and a constitutional isomer of Tonalide®, with a quite similar smell.
Synthesis precursor :
Tonalide® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Tonalide® is a polycyclic musk, synthesized from 1,1,2,4,4,7-hexamethyltetralin. This compound is synthesized by a reaction between alpha-para-dimethylstyrene and tetramethylethene, in an acid medium. It can also be prepared by a Friedel-Crafts reaction between para-Cymene and 3,3-dimethyl-1-butene in the presence of aluminium chloride. The last synthesis step is an acetylation with chloroacetic acid in the presence of aluminium chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

