Photo credits: ScenTree SAS
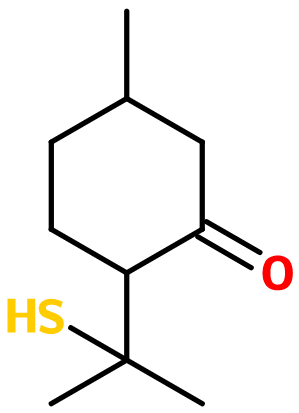
Thiomenthone®
Blackcurrant Body ; Blackcurrant Mercaptan® ; 5-methyl-2-(2-sulfanylpropan-2-yl)cyclohexan-1-one ; Buchu ketone ; Buchu mercaptan ; 2-(1-mercapto-1-methylethyl)-5-methyl-cyclohexanone ; Jallione ; Mangone ; Para-mentha-8-thiol-3-one ; P-menthane-8-thiol-3-one ; P-menthon-8-thiol ; 8-mercapto-3-p-menthanone ; Mercaptoisopropyl-5-methylcyclohexanone ; Thiomenthone ; Ribes mercaptan ; Pulegone mercaptan ; 5-methyl-2-(1-methyl-1-sulfanylethyl)cyclohexanone

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Corps Cassis - 30gr | - |
Visit website
|
- | - | - | - | - | - | |
|
|
P-MENTHA-8-THIOL-3-ONE | 441M011000 |
Visit website
|
Synthetic Aroma Chemicals |

|
- | - | - | UK | - |
General Presentation
-
CAS N° : 38462-22-5
-
EINECS number : 253-953-1
-
FEMA number : 3177
-
FLAVIS number : 12.038
-
JECFA number : 561
-
Appearance : Colorless liquid
-
Density : 1
-
Volatility : Head
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C10H18OS
-
Molecular Weight : 186,32 g/mol
-
Log P : 2,45
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 56°C (à 0,1 mmHg)
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 108°C
Uses
Uses in perfumery :
Thiomenthone® is used in grapefruit accords, exotic fruits, green, white flowers, red fruits and peach notes. Provides a powerful head note.
Year of discovery :
1968
Natural availability :
Thiomenthone® is present in Buchu EO in its natural state, in very small quantities.
Isomerism :
The asymmetric carbon of Thiomenthone® gives rise to two enantiomers (R) and (S). However, it is the racemic mixture of the two enantiomers that is used in perfumery.
Synthesis precursor :
Thiomenthone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Thiomenthone® is synthesized by reaction between Pulegone and hydrogen sulfide in the presence of a base such as triethylamine.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

