Photo credits: ScenTree SAS
Stemone®
Tremone® ; Heptoxime® ; Oxime EAC® ; N-(5-methylheptan-3-ylidene)hydroxylamine ; Ethyl 2-methylbutyl ketoxime ; Greenone ; N-hydroxy-5-methylheptan-3-imine ; Leafy oxime ; 5-methyl heptan-3-one oxime ; Methyl heptanone oxime ; Stigemone ; Tremone ; Vertone

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Stemone - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 22457-23-4
-
EINECS number : 245-010-8
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,888
-
Volatility : Heart
-
Price Range : €€
Physico-chemical properties
-
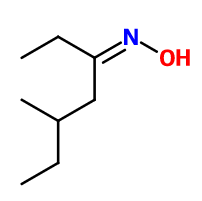
Molecular formula : C8H17NO
-
Molecular Weight : 143,23 g/mol
-
Log P : 2,3
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 95°C
Uses
Uses in perfumery :
Stemone® is used in all types of fragrances, for notes of fig leaf, tomato leaf and blackcurrant. Brings a leafy and stem facet to floral notes in particular. Also brings a green facet to a violet. Gives a natural and modern effect.
Year of discovery :
1967
Natural availability :
Stemone® is not available in its natural state.
Isomerism :
Stemone® has an asymmetric carbon and a double bond that gives rise to four possible isomers. Nevertheless, it is the mixture of these isomers that is used in perfumery.
Synthesis precursor :
Stemone® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Stemone® is part of a family of molecules called oximes. It is therefore prepared by oximation of 5-methyl-3-heptanone. This reaction corresponds to the condensation of ketone with hydroxylamine.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

