Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 33885-51-7
-
EINECS number : 251-717-2
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Viscous yellow liquid
-
Density : 0,96
-
Volatility : Heart/Base
-
Price Range : €€€
Physico-chemical properties
-
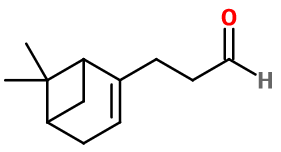
Molecular formula : C12H18O
-
Molecular Weight : 178,27 g/mol
-
Log P : 3,53
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 89°C
Uses
Uses in perfumery :
Pino Acetaldehyde gives a green facet to some fruity notes such as pineapple, and gives a ''shiny '' effect in every kind of perfumes.
Year of discovery :
1957
Natural availability :
Pino Acetaldehyde is not available in its natural state.
Isomerism :
Pino Acetaldehyde incudes two asymmetric carbons and one double bond, all included in a bicyclic group. This can only give birth to a unique isomeric form. Then, only one of the Pino Acetaldehyde isomers is used in perfumery. On another hand, Pino Acetaldehyde is a constitutional isomer of Phenoxanol®, even if it does not have the same smell at all.
Synthesis precursor :
Pino Acetaldehyde is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
The synthesis of Pino Acetaldehyde starts from alpha-Pinene. An epoxidation reaction between this molecule and peracetic acid gives birth to an intermediary called Pinocarveol, after a rearrangement of the epoxyde. An addition reaction between Pinocarveol and Ethyl Vinyl Ether forms another intermediary, able to undergo a Claisen rearrangement by being heated, giving birth to the final product, Pino Acetaldehyde.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

