Photo credits: ScenTree SAS
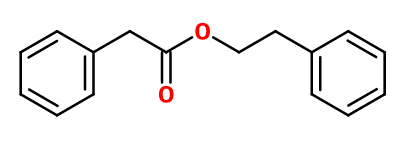
Phenyl ethyl phenyl acetate
Phenyl Ethyl Penyl acetate ; 2-Phenyl Ethyl-2-phenylacetate ; Benzyl carbinyl alpha-toluate ; Benzyl carbinyl phenyl acetate ; Phenethyl alpha-toluate ; Phenethyl phenylacetate ; Phenylethyl benzeneacetate

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 102-20-5
-
EINECS number : 203-013-1
-
FEMA number : 2866
-
FLAVIS number : 09.707
-
JECFA number : 999
-
Appearance : White solid
-
Density : 1,082
-
Volatility : Base
-
Price Range : €
Physico-chemical properties
-
Molecular formula : C16H16O2
-
Molecular Weight : 240,3 g/mol
-
Log P : 3,93
-
Fusion Point : 28°C
-
Boiling Point : 325°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 113°C
Uses
Uses in perfumery :
Phenyl Ethyl Phenyl acetate is used in all types of perfumery for rosy or white floral notes. Allows to bring tenacity and a natural effect to the note.
Year of discovery :
Data not available.
Natural availability :
Phenyl Ethyl Phenyl acetate is present in Champaca Absolute and can be extracted in its natural state but at a very high cost. Therefore, Phenyl Ethyl Phenyl acetate is greatly produced by organic synthesis.
Isomerism :
Phenyl Ethyl Phenyl acetate does not have any isomer used in perfumery.
Synthesis precursor :
Phenyl Ethyl Phenyl acetate is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Phenyl Ethyl Phenyl acetate is synthesized by an esterification reaction between Phenylacetic Acid (obtained from benzyl chloride and sodium cyanide) and Phenyl Ethyl Alcohol (obtained by a Friedel-Craft reaction between benzene and ethylene chloride). This reaction is catalysed by a concentrated sulfuric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

