Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
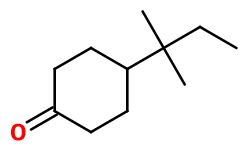
CAS N° : 16587-71-6
-
EINECS number : 240-642-0
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,92
-
Volatility : Heart
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C11H20O
-
Molecular Weight : 168,28 g/mol
-
Log P : 3,4
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 104°C
Uses
Uses in perfumery :
Orivone® is used in majority in orris root accords. It can also be used in nutty, dry woods and leather accords, to bring a dry effect and an orris root and powdery note in small quantity.
Year of discovery :
1950
Natural availability :
Orivone® is not found in nature.
Isomerism :
Orivone® is a constitutional isomer of Ethyl Linalool and so called Aldehyde C11 Lenic, but has no common facet with these two molecules.
Synthesis precursor :
Orivone® is not a precursor to the synthesis of any other molecule used in perfumery.
Synthesis route :
Orivone® can be synthesized in one step from 4-tert-amylphenol, by a selective hydrogenation reaction, catalyzed by a heterogeneous catalysor such as palladium on alumina. 4-tert-amylphenol can be taken as a starting reagent, but it can also be synthesized first by an selective alkylation reaction from phenol, on a para position, reacting with amyl chloride, with a Lewis acid catalysor.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,027 % 0,0080 % 0,16 % 0,15 % 0,038 % 0,038 % 0,038 % 0,013 %0,061 % Cat.5A B C DCat.6 0,038 % 0,038 % 0,038 % 0,013 %0,061 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,24 % 0,24 %0,013 % 0,29 % 0,061 % 1,1 %0,013 % 0,013 %61 % Cat.10A BCat.11A BCat.12 0,061 % 1,1 %0,013 % 0,013 %61 %


