Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
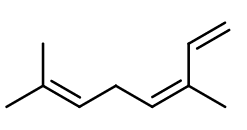
CIS-OCIMENE | M_0052890 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 13877-91-3
-
EINECS number : 237-641-2
-
FEMA number : 3539
-
FLAVIS number : 01.018
-
JECFA number : 1338
-
Appearance : Colorless to amber liquid
-
Density : 0,801
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C10H16
-
Molecular Weight : 136,24 g/mol
-
Log P : 4,8
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 66°C (à 17 hPa)
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 56°C
Uses
Uses in perfumery :
Ocimene is used in all types of perfumes, for lavender, floral-green (gardenia), mushroom and fruity-mango notes.
Year of discovery :
Data not available.
Natural availability :
Ocimene is present in many plants from which it can be extracted. Lavender EO is famous for its Ocimene, as each species does not have the same proportion. Lavender Matherone is a clonal lavender that contains 15% Ocimene and is therefore ideal to isolate the molecule. Nevertheless, synthetic Ocimene is most often used in perfumery.
Isomerism :
Ocimene has a double bond responsible for the existence of two diastereomers of the molecule. The cis isomer is more grassy and the trans isomer is closer to mushrooms. The racemic mixture is most often used in perfumery. Another isomer called Allocimene exists and has a fresher, herbaceous and more terpenic smell than the racemic classic Ocimene. D-Limonene, Myrcene, Pinene and Terpinene are constitutional isomers of Ocimene, and are part of the family of terpenes, although their smell is very far from mushroom and Ocimene.
Synthesis precursor :
Ocimene can be used for the synthesis of other terpenes by a Diels-Alder reaction, or can be hydrogenated to obtain other compounds of olfactory interest.
Synthesis route :
Ocimene is a terpene obtained by a pyrolysis of alpha-Pinene. This process simply involves heating the molecule (around 200°C) in order to break a particular bond, thereby forming a new compound.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

