Photo credits: ScenTree SAS
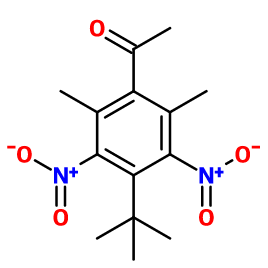
Musk ketone
1-(4-tert-butyl-2,6-dimethyl-3,5-dinitrophenyl)ethanone, 1-acetyl-4-(tert-butyl)-2,6-dimethyl-3,5-dinitrobenzene, 4-tert-butyl-2,6-dimethyl-3,5-dinitroacetophenone, 1-(4-tert-butyl-2,6-dimethyl-3,5-dinitrophenyl)ethanone, 4-tert-butyl-3,5-dinitro-2,6-dimethylacetophenone, 1-(4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl)ethanone, 1-(4-(1,1-dimethylethyl)-2,6-dimethyl-3,5-dinitrophenyl)-ethanone, ketone moschus, muskketone

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 81-14-1
-
EINECS number : 201-328-9
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Yellow powder
-
Density : 1,205
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C14H18N2O5
-
Molecular Weight : 294,31 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : 135°C
-
Boiling Point : 395°C
-
Detection Threshold : 0,1 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 100°C
Uses
Uses in perfumery :
Musk Ketone is used in women perfumes for a powdery effect. Goes on well with white flowers accords.
Solubilization issues in alcohol.
Mostly used in fine fragrance, less and less beacause of its vintage connotation.
Year of discovery :
Patented in 1893 by Willem Mallmann and transferred to the Laire factories in 1896. The reason? W. Mallmann illegally produced this compound chemically very close to musk Xylene, itself patented by the Fabriques DeLaire in 1888.
Natural availability :
Musk Ketone is not available in its natural state.
Isomerism :
Musk Ketone is a nitrated musk with no isomer used in perfumery.
Synthesis precursor :
Musk Ketone is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Musk Ketone is synthesized from 1,3-dimethyl-5-tert-butylbenzene, by a Friedel-Craft acetylation, with chloroacetic acid and in the presence of aluminum chloride. This reaction is followed by a nitration of the obtained compound with nitric acid.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is restricted by the 51th amendment
-
Specified ingredients: notes
Musk xylene (CAS number 81-15-2), which has been prohibited for use in fragrance compounds for environmental reasons (vPvB), can be present in Musk ketone as an impurity. Musk ketone should only be used if it contains less than 0.1% of Musk xylene.


