Photo credits: ScenTree SAS
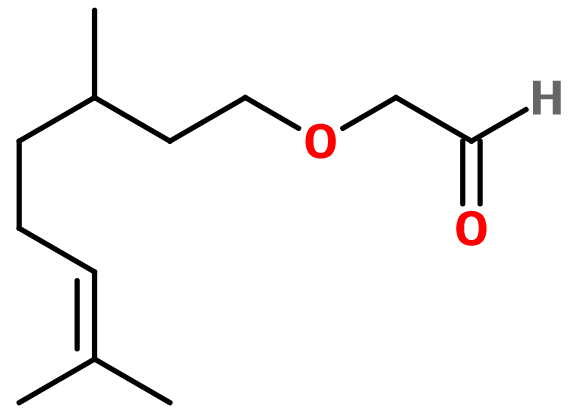
Muguet aldehyde
((3,7-dimethyl-6-octenyl)oxy)-acetaldehyde ; Citronellyloxyacetaldehyde ; Citronellyloxy Acétaldehyde ; Citronelloxyacetaldehyde ; Muguet aldehyde ; 6,10-dimethyl-3-oxa-9-undecenal ; (3,7-dimethyl-6-octenyl) oxyacetaldehyde

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
CITRONELLYLOXY ACETALDEHYDE | 85160 |
Visit website
|
Molecule | - | - | - | - | - |
General Presentation
-
CAS N° : 7492-67-3
-
EINECS number : 231-324-2
-
FEMA number : 2310
-
FLAVIS number : 05.079
-
JECFA number : 593
-
Appearance : Colorless liquid
-
Density : 0,97
-
Volatility : Head/Heart
-
Price Range : Data not available.
Physico-chemical properties
-
Molecular formula : C12H22O2
-
Molecular Weight : 198,31 g/mol
-
Log P : 3,26
-
Fusion Point : Donnée indisponible.
-
Boiling Point :
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : >94°C
Uses
Uses in perfumery :
Muguet Aldehyde is used in floral-aldehydic and green notes of lily of the valley, to bring an aldehydic effect. It is over all used in functional perfumery.
Year of discovery :
Data not available.
Natural availability :
Muguet Aldehyde is not found on a natural state.
Isomerism :
As Citronellol, Muguet Aldehyde has one asymetric carbon. A mixture of its two enantiomers is anyway used in perfumery. It also is a constitutional isomer of Citronellyl acetate, having a close structure, but being weigh fruitier.
Synthesis precursor :
Muguet Aldehyde is not a precursor for the synthesis of another compound of olfactive interest.
Synthesis route :
Muguet Aldehyde is prepared by interaction between bromoacetals with sodium or potassium alcoholates.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

