Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
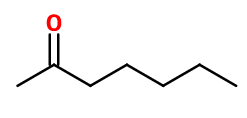
CAS N° : 110-43-0
-
EINECS number : 203-767-1
-
FEMA number : 2544
-
FLAVIS number : 07.002
-
JECFA number : 283
-
Appearance : Colorless liquid
-
Density : 0,82
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C7H14O
-
Molecular Weight : 114,18 g/mol
-
Log P : 1,98
-
Fusion Point : -35°C
-
Boiling Point : 150°C
-
Detection Threshold : 1 ppb et 1,33 ppm
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 46°C
Uses
Uses in perfumery :
Methyl Amyl Ketone is used in fruity notes, lavender notes (in trace amounts to give power from the top) and spicy notes.
Year of discovery :
Data not available.
Natural availability :
Methyl Amyl Ketone is present in many plants and cheeses. The plant that contains the most is a green alga called Ulva Rigida. However, it is the synthetic Methyl Amyl Ketone which is most often used in perfumery.
Isomerism :
When the ketone function of Methyl Amyl Ketone is relocated, the smell can radically change. For example, Ethyl Butyl Ketone has a greener, oily and fruity smell. Aldehyde C-7 is a constitutional isomer of Methyl Amyl Ketone, but has a totally different smell from the latter.
Synthesis precursor :
A hydrogenation of Methyl Amyl Ketone converts it to 2-heptanol, which also has an olfactory interest.
Synthesis route :
Methyl Amyl Ketone is synthesized by a catalytic oxidation of 2-heptenol. This oxidation can be done in the presence of sodium hypochlorite at a high temperature, for example.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

