Photo credits: ScenTree SAS
Methyl Pamplemousse®
Grapefruit Acetal ; Amarocite® ; 6,6-dimethoxy-2,5,5-trimethylhex-2-ene ; 1,1-dimethox-2,2,5-trimethy-4-hexene ; 1,1-dimethoxy-2,2,5-trimethyl hex-4-ene ; 6,6-dimethoxy-2,5,5-trimethyl-2-hexene ; Methyl pomello ; Pamplerom ; Pomelocit

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Méthyl Pamplemousse® - 30gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 67674-46-8
-
EINECS number : 266-885-2
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,877
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
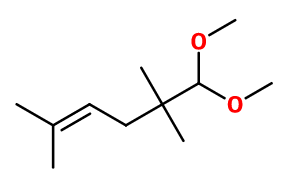
Molecular formula : C11H22O2
-
Molecular Weight : 186,29 g/mol
-
Log P : 4,3
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 215°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 75°C
Uses
Uses in perfumery :
Methyl Pamplemousse® is used in all types of perfumes, in eau fraiche and soaps especially (for its stability), for notes of grapefruit. Gives naturalness to a grapefruit accord. Used in fougere, chypre and fruity notes to boost the head. Brings modernity to citrus fruits.
Year of discovery :
1983
Natural availability :
Methyl Pamplemousse® is not available in its natural state.
Isomerism :
Isononyl acetate is a constitutional isomer of Methyl Pamplemousse®. These two compounds do not have any olfactive similarities.
Synthesis precursor :
Methyl Pamplemousse® is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Methyl Pamplemousse® is synthesized by an acetalization reaction between 2,2,5-trimethyl-4-hexenal (obtained from isobutyraldehyde and prenyl chloride) and methanol, in the presence of calcium chloride.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

