Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
Melonal - 30 Gr | - |
Visit website
|
- | - | - | - | - | - |
General Presentation
-
CAS N° : 106-72-9
-
EINECS number : 203-427-2
-
FEMA number : 2389
-
FLAVIS number : 05.074
-
JECFA number : 349
-
Appearance : Colorless liquid
-
Density : 0,851
-
Volatility : Head
-
Price Range : €€
Physico-chemical properties
-
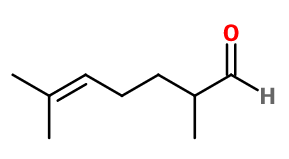
Molecular formula : C9H16O
-
Molecular Weight : 140,23 g/mol
-
Log P : 3,4
-
Fusion Point : -20°C
-
Boiling Point : 181°C
-
Detection Threshold : 1,3465 ng/l air
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 62°C
Uses
Uses in perfumery :
Melonal® is used in all types of perfumes for marine notes, juicy fruits (especially melon and cucumber reproduction), citrus and foral notes.
Year of discovery :
Patent N°4,242,281 (US) published on Oct. 26, 1979 for IFF company
Natural availability :
Melonal® is present in Ginger EO, from which it can be extracted in very small quantities in its natural state. There are natural qualities from white melon and Petitgrain Bigarade EO, much more expensive but more powerful and fatty.
Isomerism :
Melonal® has an asymmetric carbon, which gives rise to two possible enantiomers for this molecule. Only the racemic mixture of the two isomers is used in perfumery. Also, Melonal® is a position isomer of cis-6-Nonenal, which has a fruity note more reminiscent of cucumber, and also used to give a aqueous facet to fruity notes.
Synthesis precursor :
Melonal® is likely to form Schiff bases with Methyl Anthranilate or Indole for example. In addition, MethoxyMelonal® is an ether synthesized from Melonal®.
Synthesis route :
Melonal® is synthesized by a Darzens reaction between 6-methyl-5-hepten-2-one and ethyl chloroacetate. This reaction leads to the formation of a glycidate, which is saponified and decarboxylated to obtain Melonal®.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

