Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 27606-09-3
-
EINECS number : 248-561-2
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 1,088
-
Volatility : Head/Heart
-
Price Range : €€
Physico-chemical properties
-
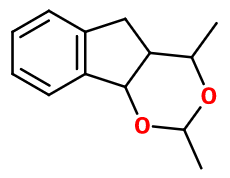
Molecular formula : C13H16O2
-
Molecular Weight : 204,27 g/mol
-
Log P : 2,7
-
Fusion Point : -40°C
-
Boiling Point : 258°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 136°C
Uses
Uses in perfumery :
Magnolan® is used for rhubarb and exotic fruits (mango) accords, floral (magnolia, geranium), citrus (grapefruit) notes, usually associated with woody and green notes. It brings modernity and roundness to citrus notes.
Year of discovery :
1967
Natural availability :
Magnolan® can't be found in nature. It can't be used as a plant extract.
Isomerism :
Magnolan® has four asymmetric carbons. Nevertheless, a mixture of its diastereoisomers is used in perfumery. Cis-3-Hexenyl Benzoate is a constitutional isomer of Magnolan®. Although, these two compounds don't have the same smell.
Synthesis precursor :
Magnolan® is not a precursor for the synthesis of another compound of olfactive interest.
Synthesis route :
Magnolan® can be synthesized by reacting Indene with Acetaldehyde.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

