Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 61792-11-8
-
EINECS number : 263-214-5
-
FEMA number : Donnée indisponible.
-
FLAVIS number : Donnée indisponible.
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,87
-
Volatility : Base
-
Price Range : €€€
Physico-chemical properties
-
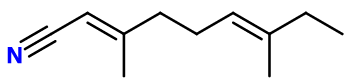
Molecular formula : C11H17N
-
Molecular Weight : 163,26 g/mol
-
Log P : 3,9
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 247°C
-
Detection Threshold : Donnée indisponible.
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 78°C
Uses
Uses in perfumery :
Lemonile® is used in lemon, verbena and lime accords, to bring freshness and diffusivity.
Year of discovery :
Data not available.
Natural availability :
Lemonile® is not found in nature. It is thus impossible to extract it on a natural state.
Isomerism :
Lemonile® is in reality a mixture of three positional isomers. Indeed, the double bond closest to the nitrile function of the molecule is delocalizable, forming three possible molecules around the ramified carbon. The other double bond has a trans (E) conformation.
Synthesis precursor :
Lemonile® is not a precursor for the synthesis of another compound of olfactive interest.
Synthesis route :
Lemonile® can be synthesized in two steps, the first one being a Knoevenagel condensation of cyanoacetic acid on 6-methyloct-5-en-2-one, with pyridine and in toluene (as a solvent). This reaction is then followed by in situ decarboxylation, meaning that it occurs by itself.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

