Photo credits: ScenTree SAS
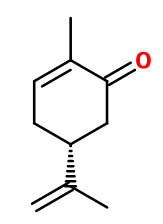
L-carvone
(5R)-2-methyl-5-prop-1-en-2-ylcyclohex-2-en-1-one ; (5R)-2-methyl-5-(1-methylethenyl)-2-cyclohexen-1-one ; L-p- mentha-1(6),8-dien-2-one ; L-para- mentha-6,8-dien-2-one ; 6,8,9-para- menthadien-2-one ; L-1-methyl-4-isopropenyl-6-cyclohexen-2-one ; (R)-2- methyl-5-(1-methyl ethenyl)-2-cyclohexen-1-one

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
laevo-CARVONE ex Mentha 97 | 3526533012 |
Visit website
|
Natural Aroma Chemicals |

|
- | - | - | France | - |
General Presentation
-
CAS N° : 6485-40-1
-
EINECS number : 229-352-5
-
FEMA number : 2249
-
FLAVIS number : 07.147
-
JECFA number : Donnée indisponible.
-
Appearance : Colorless liquid
-
Density : 0,959
-
Volatility : Head/Heart
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C10H14O
-
Molecular Weight : 150,22 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 229°C
-
Detection Threshold : 30 ppb environ (0,000003%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 94°C
Uses
Uses in perfumery :
L-Carvone is used in mint reconstitutions, for its fidelity to this raw material and for a vegetal-green minty note.
Year of discovery :
Data not available.
Natural availability :
L-Carvone is obtained in its natural state from Spearmint EO, of which it is the majority compound (between 55 and 75% according to the varieties).
Isomerism :
L-Carvone is an enantiomer of D-Carvone, the main constituent of Caraway EO, with a less minty and much more spicy smell. Dimethyl Benzyl Carbinol and Thymol are constitutional isomers of L-Carvone. Their smell, more floral for one and more aromatic for the other, is very different from Carvone.
Synthesis precursor :
L-Carvone can be a precursor to the synthesis of other terpenes, by a Diels-Alder reaction for example.
Synthesis route :
Originally, L-Carvone was separated from D-Carvone from Spearmint EO. Today, its synthesis is made from D-Limonene. The synthesis process reverses the optical activity of the final product. A first reaction consists of reacting D-Limonene with nitrosyl chloride. The resulting D-Limonene nitrosochloride is subjected to a treatment with a weak base, removing hydrochloric acid. Finally, an acid hydrolysis in the presence of acetone allows to obtain the final product. Another method synthesizes L-Carvone from the same D-Limonene, transforming it into its 1,2-epoxide, resulting in a regioselective rearrangement, making it possible to obtain L-Carveol. The catalysis of this first step is metallic and phenolic. An Oppenhauer oxidation allows to obtain the final L-Carvone. The advantage of this synthesis is that it is achievable inside of an unique reactor.
Regulations & IFRA
Allergens :
This ingredient is classified as an allergen under European Regulation 2023/1545, dated August 26, 2023.
Its presence must therefore be declared on product labels when it exceeds 0.001% in leave-on products and 0.01% in rinse-off products.
IFRA 51th :
This ingredient is restricted by the 51th amendment
- Quantitative limit on the use :
-
Cat.1 Cat.2 Cat.3 Cat.4 Cat.5A B C DCat.6 0,2 % 0,06 % 0,02 % 0,59 % 0,2 % 0,039 % 0,059 % 0,013 %0,66 % Cat.5A B C DCat.6 0,2 % 0,039 % 0,059 % 0,013 %0,66 % Cat.7A BCat.8 Cat.9 Cat.10A BCat.11A BCat.12 0,039 % 0,039 %0,013 % 0,18 % 0,18 % 0,43 %0,013 % 0,013 %17 % Cat.10A BCat.11A BCat.12 0,18 % 0,43 %0,013 % 0,013 %17 %
Annexe I :
Some regulated synthetic ingredients are found in nature and in certain proportions in natural ingredients. This presence in nature has to be taken into account when calculating limits of use recommended by the IFRA. In case you do not know these concentrations, you can use the ones estimated by the IFRA. Here they are :
| List of regulated compounds contained in this ingredient | |||
|---|---|---|---|
| Ingredient Name | Botanical Name | CAS N° | Estimated Concentration |
| Yarrow oil | Achillea millefolium L. | 8022-07-9 | 0,2 |
| Basil oil, chemotype estragole | Ocimum basilicum L. | 8015-73-4 | 0,49 |
| Carrot seed oil | Daucus carota L. | 8015-88-1 | 0,05 |
| Cistus oil | Cistus ladaniferus L. | 8016-26-0 | 0,6 |
| Lovage root oil | Levisticum officinale Koch | 8016-31-7 | 0,02 |
| Mentha arvensis oil | Mentha arvenis L. | 68917-18-0 | 0,25 |
| Spearmint oil, Scotch | Mentha x gentilis L. syn. Mentha x gracilis Sole | 64,12 | |
| Spearmint oil | Mentha spicata L. | 8008-79-5 | 66,07 |
| Myrtle oil | Myrtus communis L. | 8008-46-6 | 0,2 |
| Parsley herb oil | Petroselinum crispum (Mill.) Nyman ex A.W.Hill | 8000-68-8 | 0,2 |
| Pine Scotch, resinoid | Pinus sylvestris L. | 0,001 | |
| Thyme absolute | Thymus vulgaris L. | 8007-46-3 | 0,05 |
| Erospicata oil, Mentha spicata 'Erospicata' | Mentha spicata Erospicata | 917-841-2 | 67 |
| Lavender oil dentata | Lavandula dentata | 93165-50-5 | 0,3 |
| Spearmint oil terpenes | Mentha spicata L. syn Mentha viridis L. var. crispa Benth. | 8,51 | |
-
Contributions from other sources
The natural contribution of Carvone is determined by the sum of the natural contributions of each of its isomers.


