Photo credits: ScenTree SAS
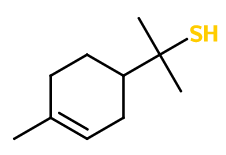
Grapefruit Mercaptan
Grapefruit Mercaptan ; Menthenethiol ; 2-(4-methyl-1-cyclohex-3-enyl)propane-2-thiol ; Para-menth-1-ene-8-thiol ; 1-para-menthen-8-thiol ; Para-menthene thiol ; Terpinyl mercaptan ; Alpha,alpha,4-trimethyl cyclohex-3-ene-1-methane thiol

Photo credits: ScenTree SAS
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
Do you sell any of the raw materials? Would you like to let our users know?
Send an email to fournisseurs@scentree.coto learn about our advertising opportunities.
General Presentation
-
CAS N° : 71159-90-5
-
EINECS number : 275-223-1
-
FEMA number : 3700
-
FLAVIS number : 12.085
-
JECFA number : 523
-
Appearance : Colorless liquid
-
Density : 1,025
-
Volatility : Head
-
Price Range : €€€€
Physico-chemical properties
-
Molecular formula : C10H18S
-
Molecular Weight : 170,32 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 110°C (à 2 mmHg)
-
Detection Threshold : Le Sulfure de limonène possède l'un des seuils de détection les plus faibles de la parfumerie : 0,00005 ppb. (0,000000000005%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 75°C
Uses
Uses in perfumery :
Grapefruit Mercaptan is used in sulfuric, fruity-exotic and vegetable notes. Also used in colognes and eaux fraiches as a booster. Often diluted directly in D-Limonene by suppliers.
Year of discovery :
Data not available.
Natural availability :
Natural Grapefruit Mercaptan can be extracted from Grapefruit EO. However, synthetic Grapefruit Mercaptan is most often used in perfumery.
Isomerism :
Grapefruit Mercaptan has an asymmetric carbon. However, the distinction between the two possible enantiomers is not made in perfumery. It is the mixture of the two isomers that is used in majority. The isomer (R) of the molecule is considered more powerful, as it has an extremely low detection limit of 200 nanograms/kg, against 800 for the isomer (S).
Synthesis precursor :
Grapefruit Mercaptan is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Grapefruit Mercaptan is synthesized in an alkaline medium, by reaction between hydrogen sulfide and the corresponding alcene, derived from para-Menthene.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

