Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
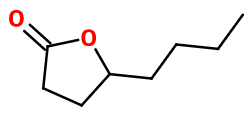
(S) GAMMA OCTALACTONE | M_0053333 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 104-50-7
-
EINECS number : 203-208-1
-
FEMA number : 2796
-
FLAVIS number : 10.022
-
JECFA number : 226
-
Appearance : Colorless liquid
-
Density : 0,98
-
Volatility : Base
-
Price Range : €€
Physico-chemical properties
-
Molecular formula : C8H14O2
-
Molecular Weight : 142,2 g/mol
-
Log P : Donnée indisponible.
-
Fusion Point : Donnée indisponible.
-
Boiling Point : 234°C
-
Detection Threshold : La Gamma-octalactone possède un seuil de détection très faible, de l'ordre du ppb, de l'ordre de 0,0000001%
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 111°C
Uses
Uses in perfumery :
Gamma-Octalactone is used in peach and coconut reconstitutions for its milky note, and in heavy floral accords as tuberose and jasmine.
Year of discovery :
Data not available.
Natural availability :
Gamma-Octalactone is found in the odoriferous principle of some processed and unprocessed food (apricot, peach, pineapple, blue cheese…), but does not exist on a natural state.
Isomerism :
Gamma-Octalactone has an asymmetric carbon, giving birth to two possible enantiomers. Nevertheless, a blend of these enantiomers is used in perfumery. Delta-Octalactone is a constitutional isomer of this molecule, but has a more woody and less milky note.
Synthesis precursor :
Gamma-Octalactone is not used for the synthesis of another molecule of olfactive interest.
Synthesis route :
Gamma-Octalactone is a cyclic lactone, synthesized as every other lactones. A reaction between acrylic acid and pentanol, in the presence of an alkaline sulfate or phosphate, ables to synthesize this molecule. An intramolecular esterification of 4-hydroxyoctanoic acid, catalized by a strong acid as sulfuric acid, also ables to obtain this compound. Eventually, biochemical synthesis routes are being studied.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

