Photo credits: ScenTree SAS
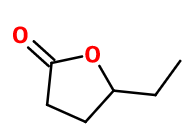
Gamma-hexalactone
5-ethyloxolan-2-one ; Gamma- caprolactone ; Dehydrotonkalide ; 4-ethyl butan-4-olide ; Gamma-ethyl butyrolactone ; 5-ethyltetrahydro-2-furanone ; 4-ethyl-4-butanolide ; 5-ethyl-dihydrofuran-2-one ; 4-ethylbutan-4-olide ; Hexan-4-olide ; Hexano-1,4-lactone ; 4-hexanolide ; 4- hydroxyhexanoic acid lactone ; Tonkalide

Photo credits: ScenTree SAS
| Company | Ingredient Name | ID | Comments | Naturality | Certifications | Purity | Latin name | Treated part | Geographical origin | MOQ |
|---|---|---|---|---|---|---|---|---|---|---|
|
|
(S) GAMMA HEXALACTONE | M_0053638 |
Visit website
|
Naturel | - | - | - | - | - |
General Presentation
-
CAS N° : 695-06-7
-
EINECS number : 211-778-8
-
FEMA number : 2556
-
FLAVIS number : 10.021
-
JECFA number : 223
-
Appearance : Colorless liquid
-
Density : 1,028
-
Volatility : Head/Heart
-
Price Range : €€€
Physico-chemical properties
-
Molecular formula : C6H10O2
-
Molecular Weight : 114,14 g/mol
-
Log P : 0,34
-
Fusion Point : -18°C
-
Boiling Point : 220°C
-
Detection Threshold : 1,6 ppm (0,00016%)
-
Optical rotation : Donnée indisponible
-
Vapor pressure : Donnée indisponible
-
Refractive Index @20°C : Donnée indisponible
-
Acid Value : Donnée indisponible.
-
Flash Point : 102°C
Uses
Uses in perfumery :
Gamma-Hexalactone is used for a gourmet, sunny and milky tonality and to recreate an exoticism in fruity notes in particular.
Year of discovery :
Data not available.
Natural availability :
Gamma-Hexalactone is present in several fruits. However, it is synthetic Gamma-Hexalactone which is more often used in perfumery.
Isomerism :
The asymmetric carbon of Gamma-Hexalactone is responsible for the presence of two enantiomers of this molecule. The (R) enantiomer of the molecule is greener than the other isomer, more woody.
Synthesis precursor :
Gamma-Hexalactone is not a precursor to the synthesis of another compound of olfactory interest.
Synthesis route :
Like some other lactones, Gamma-Hexalactone can be synthesized by an intramolecular esterification reaction, using 4-hydroxyhexanoic acid and concentrated sulfuric acid, for example, in a catalytic amount.
Regulations & IFRA
Allergens :
This ingredient does not contain any allergen.
IFRA 51th :
This ingredient is not restricted for the 51th amendment

